| |
13:30
|
0646.
 |
A 3-Dimensional Microvascular Phantom for Perfusion Imaging 
Thomas Gaass1,2, Moritz Schneider1,
Michael Ingrisch1, Julia Herzen3, and
Julien Dinkel1,2
1Institute for Clinical Radiology, Ludwig-Maximilians
University, Munich, Germany, 2Comprehensive
Pneumology Center, German Center for Lung Research, Munich,
Germany, 3Department
of Physics, Technische Universität München, Munich, Germany
The presented work demonstrates the applicability of a
dedicated 3-dimensional phantom as a realistic MR- and
CT-compatible phantom for microvascular perfusion
simulation. The device constructed using resin-embedded,
melt-spun, sacrificial sugar structures was examined using
dynamic contrast enhanced MRI. Parameters, such as flow and
volume fraction gained from deconvolving the signal
enhancement curve showed very good agreement with the
pre-set perfusion parameters. The presented phantom showed
great potential in realistically simulating the capillary
bed and can potentially serve as a quality insurance device
for quantitative dynamic contrast enhanced MRI in the
future.
|
| |
13:42
|
0647.
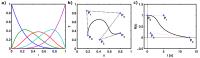 |
Bézier Curve Deconvolution for Model-Free Quantification of
Cerebral Perfusion 
André Ahlgren1, Ronnie Wirestam1,
Freddy Ståhlberg1,2,3, and Linda Knutsson1
1Department of Medical Radiation Physics, Lund
University, Lund, Sweden, 2Department
of Diagnostic Radiology, Lund University, Lund, Sweden, 3Lund
University Bioimaging Center, Lund University, Lund, Sweden
Deconvolution is an ill-posed and ill-conditioned inverse
problem that often yields non-physiological residue
functions in perfusion MRI. Deconvolution methods based on
Fourier transform or matrix decomposition often yield
solutions with spurious oscillations. Although the perfusion
value, estimated from the peak of the tissue impulse
response function, may still be useful, any estimate that
depends on the actual shape of the residue function will be
prone to errors. To obtain physiologically reasonable
residue functions in perfusion MRI, we propose the use of
Bézier curves, and demonstrate initial experiences from the
application to DSC-MRI in vivo data.
|
| |
13:54
|
0648.
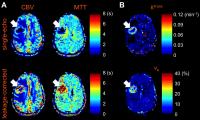 |
Simultaneous perfusion and permeability assessments using
multi-band multi-echo EPI (M2-EPI) 
Deqiang Qiu1, Junjie Wu1, Seena
Dehkharghani1, and Amit Saindane1
1Department of Radiology and Imaging Sciences,
Emory University, Atlanta, GA, United States
We proposed a novel multi-band multi-echo DSC perfusion
imaging method to estimate leakage-corrected perfusion
parameters and additional vascular permeability parameters.
Simulations were performed and showed that higher temporal
resolution provided by the novel sequence improves the
accuracy in the calculation of perfusion parameters.
|
| |
14:06
|
0649.
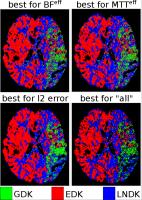 |
Unveiling the Dispersion Kernel in DSC-MRI by Means of
Dispersion-Compliant Bases and Control Point Interpolation
Techniques 
Marco Pizzolato1, Rutger Fick1,
Timothé Boutelier2, and Rachid Deriche1
1Athena Project-Team, Inria Sophia Antipolis -
Méditerranée, Sophia Antipolis, France, 2Olea
Medical, La Ciotat, France
In DSC-MRI the presence of dispersion affects the
estimation, via deconvolution, of the residue function that
characterizes the perfusion in each voxel. Dispersion is
described by a Vascular Transport Function (VTF) which
knowledge is essential to recover a dispersion-free residue
function. State-of-the-art techniques aim at characterizing
the VTF but assume a specific shape for it, which in reality
is unknown. We propose to estimate the residue function
without assumptions by means of Dispersion-Compliant Bases
(DCB). We use these results to find which VTF model better
describes the in-vivo data for each tissue type by means of
control point interpolation approaches.
|
| |
14:18
|
0650.
 |
Arterial transit time (ATT) heterogeneity in calf muscle: how
DCE studies reveal a critical challenge for arterial spin
labeling (ASL) acquisition 
Jeff L. Zhang1, Christopher Hanrahan1,
Christopher C. Conlin1, Corey Hart2,
Gwenael Layec2, Kristi Carlston1,
Daniel Kim1, Michelle Mueller3, and
Vivian S. Lee1
1Radiology, University of Utah, Salt Lake City,
UT, United States, 2Internal
Medicine, University of Utah, Salt Lake City, UT, United
States, 3Vascular
surgery, University of Utah, Salt Lake City, UT, United
States
One major challenge for measuring exercise perfusion of
skeletal muscle with ASL is the potential heterogeneity of
arterial transit time (ATT) across the muscle. In this
study, we used reliable DCE MRI technique to measure ATT of
calf muscle in both healthy controls and peripheral artery
disease patients and after plantar flexion of different
loads. Our study showed that ATT of calf muscle varied with
multiple factors, including muscle group, exercise load and
healthy status, and had a wide range of 0~4 sec. The result
suggests the necessity of performing calf-muscle ASL with
multiple different post-labeling delays.
|
| |
14:30
|
0651.
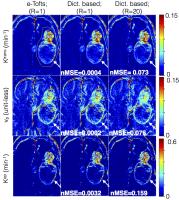 |
Accelerated brain DCE-MRI using Contrast Agent Kinetic Models as
Temporal Constraints 
Sajan Goud Lingala1, Yi Guo1, Yinghua
Zhu1, Naren Nallapareddy1, R. Marc
Lebel2, Meng Law3, and Krishna Nayak1
1Electrical Engineering, University of Southern
California, Los Angeles, CA, United States, 2GE
Health care, Calgary, Canada, 3Radiology,
University of Southern California, Los Angeles, CA, United
States
We propose a novel tracer-kinetic model based constrained
reconstruction scheme to enable highly accelerated DCE-MRI.
The proposed approach efficiently leverages information of
the contrast agent kinetic modeling into the
reconstruction, and provides a novel alternative to current
constraints that are blind to tracer kinetic modeling. We
develop the frame-work to include constraints derived from
the extended-Tofts (e-Tofts) model. We perform noise
sensitivity analysis to determine the accuracy and precision
of parameter mapping with the proposed e-Tofts derived
temporal bases. We demonstrate its utility in
retrospectively accelerating brain tumor DCE datasets with
different tumor characteristics.
|
| |
14:42
|
0652.
 |
High temporal resolution DCE MRI of breast cancer treated with
neo-adjuvant chemotherapy and analyzed with both distributed
parameter and modified Tofts tracer kinetics models 
Dennis Lai-Hong Cheong1, Bo Zhang1,
Bingwen Zheng1, Limiao Jiang1, Eugene
Wai Mun Ong2, Soo Chin Lee3,4, and
Thian C Ng1
1Clinical Imaging Research Centre, A*STAR-NUS,
Singapore, Singapore, 2Department
of Diagnostic Imaging, National University Hospital in
Singapore, Singapore, Singapore, 3Department
of Haematology-Oncology, National University Cancer
Institute, National University Health System, Singapore,
Singapore, 4Cancer
Science Institute of Singapore, Singapore, Singapore
Higher resolution DCE-MRI is readily attainable and should
allow for more realistic distributed parameter tracer
kinetics models to be used. However, simpler, faster and
lesser parameters compartmental models such as the modified
Tofts model are still preferred by many researchers. We
present here how we have implemented a distributed parameter
model to analyze 2.4sec/frame DCE MRI data in a clinical
trial of neo-adjuvant chemotherapy with or without
short-course anti-angiogenic therapy in breast cancer
patients. The results from modified Tofts and the
distributed parameter model differ. More realistic
distributed parameter models might be better in analyzing
high temporal resolution DCE-MRI data.
|
| |
14:54
|
0653.
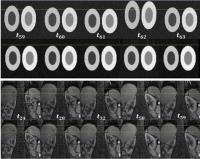 |
Motion Correction in DCE-MRI by Tracer-Kinetic Model-Driven
Registration: Beyond the Tofts models 
Dimitra Flouri1,2, Daniel Lesnic2, and
Steven P Sourbron 1
1Division of Biomedical Imaging, University of
Leeds, Leeds, United Kingdom, 2Department
of Applied Mathematics, University of Leeds, Leeds, United
Kingdom
Tracer-kinetic model-driven motion correction is an
attractive solution for DCE-MRI, but previous studies only
use the extended Tofts model. We propose a generalisation
based on a 4-parameter 2-compartment tracer-kinetic model,
and evaluate it in simulated and patient kidney data.
Results show a significantly improved alignment of the data
and removal of the motion-induced parameter error at a wide
range of noise levels. With improvement in calculation time
this is viable method for motion correction in arbitrary
DCE-MRI data.
|
| |
15:06
|
0654.
 |
Impact of fitting strategy on DCE parameter estimates and
performance : a simulation study in image space 
Charlotte Debus1, Ralf Floca2, Amir
Abdollahi1, Jürgen Debus3, and Michael
Ingrisch4
1Translational Radiation Oncology, German Cancer
Research Center (DKFZ), Heidelberg, Germany, 2Software
development for Integrated Diagnostics and Therapy, German
Cancer Research Center (DKFZ), Heidelberg, Germany, 3Department
of Radiology, University of Heidelberg Medical School,
Heidelberg, Germany, 4Institute
for Clinical Radiology, Ludwig-Maximilians-University
Hospital, Munich, Germany
The two-compartment exchange model is a tracer-kinetic model
that is defined by two coupled first-order differential
equations. These can be solved analytically or by direct
integration. In this simulation study, we compared both
strategies for different parameter scenarios in synthetic 4D
images. The sum of squared residuals was calculated either
by numeric integration with the Runge-Kutta method or by
numeric convolution. The resulting parameter estimates were
evaluated in terms of accuracy, precision and computational
speed. Both approaches yield similar results in parameter
determination, the convolution excelled in computational
speed.
|
| |
15:18
|
0655.
 |
A Novel Prostate DCE-MRI Flow Phantom for the Quantitative
Evaluation of Pharmacokinetic Parameters 
Silvin P. Knight1, Jacinta E. Browne2,
James F. Meaney1, David S. Smith3, and
Andrew J. Fagan1
1National Centre for Advanced Medical Imaging
(CAMI), St James Hospital / School of Medicine, Trinity
College University of Dublin, Dublin, Ireland, 2School
of Physics, Medical Ultrasound Group, Dublin Institute of
Technology, Dublin, Ireland, 3Institute
of Imaging Science / Department of Radiology and
Radiological Sciences, Vanderbilt University, Nashville, TN,
United States
A method is lacking to comprehensively validate and optimise
the ability of prostate DCE-MRI techniques to accurately
measure pharmacokinetic (PK) parameters. We present a novel
flow phantom capable of simultaneously producing two
measurable, reproducible, and known arbitrarily-shaped
contrast time-intensity curves, from which PK parameters can
be derived. Ktrans values
were derived from MR data acquired at different temporal
resolutions (2.3-20.3s) and were found to differ by -8.1% to
-44.6%, when compared to calibrated ‘truth estimate’ values,
with the lowest variance measured at a temporal resolution
of 6.8s. The phantom can be used to help establish robust
DCE-MRI prostate protocols.
|
|










