| |
10:00
 |
0297.
 |
Simultaneous Multi-Slice Spiral-CEST Encoding with Hankel
Subspace Learning: ultrafast whole-brain z-spectrum acquisition 
Suhyung Park1, Sugil Kim1,2, and
Jaeseok Park3
1Center for Neuroscience Imaging Research,
Institute for Basic Science (IBS), Suwon, Korea, Republic
of, 2Department
of Brain and Cognitive Engineering, Korea University, Seoul,
Korea, Republic of, 3Department
of Biomedical Engineering, Sungkyunkwan University, Suwon,
Korea, Republic of
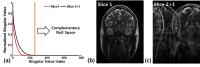
Chemical exchange saturation transfer (CEST) imaging has
been introduced as a new contrast mechanism for molecular
imaging, and typically requires long saturation preparation
and multiple acquisitions of imaging data with varying
saturation frequencies (called z-spectrum). Since the
z-spectrum acquisition is inherently slow and takes
prohibitively long imaging time, it has been very difficult
to introduce CEST z-spectrum into a clinical routine. In
this work, we propose a novel, simultaneous multi-slice (SMS)
Spiral CEST encoding with Hankel subspace learning (HSL) for
ultrafast whole-brain z-spectrum acquisition within 2-3
minutes, in which: 1) RF segmented uneven saturation is
employed to reduce the duration of saturation preparation,
2) Spiral CEST encoding is employed to acquire SMS signals,
and 3) SMS signals are projected onto the subspace spanned
by the complementary null space, selectively reconstructing
a slice of interest while nulling the other slice signals.
|
| |
10:12
|
0298.
 |
Superfast CEST Spectral Imaging (SCSI) 
Iris Yuwen Zhou1, Jinsuh Kim2,
Takahiro Igarashi1, Lingyi Wen1, and
Phillip Zhe Sun1
1Athinoula A. Martinos Center for Biomedical
Imaging, Department of Radiology, Massachusetts General
Hospital and Harvard Medical School, Charlestown, MA, United
States, 2Department
of Radiology, University of Illusions at Chicago, Chicago,
IL, United States
To resolve metabolites at different chemical shift offsets,
complete Z-spectrum is conventionally obtained by varying
saturation offset from scan to scan, which is time consuming
and not suitable for studying dynamic changes. To overcome
this, we innovatively combined superfast Z spectroscopy with
chemical shift imaging (CSI) and developed Superfast
Chemical exchange saturation transfer (CEST) Spectral
Imaging (SCSI). It provides fast Z-spectral CEST information
with spatial resolution. While conventional CSI measures
dilute metabolites, the proposed SCSI exploits CEST
mechanism to investigate the interaction between
metabolites/contrast agents and tissue water, providing
sensitivity enhanced measurements of metabolites and pH
information.
|
| |
10:24
|
0299.
 |
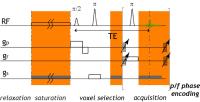
Clinically relevant rapid 3D CEST imaging with hexagonal
spoiling gradients, optimised B1, and symmetric z-spectrum
sampling 
Robert C. Brand1, Nicholas P. Blockley1,
Michael Chappell2, and Peter Jezzard1
1FMRIB, Nuffield Department of Clinical
Neurosciences, University of Oxford, Oxford, United Kingdom, 2IBME,
University of Oxford, Oxford, United Kingdom
Clinical 3D CEST has been hindered by slow acquisition times
and z-spectra artefacts that affect fitting. Here, we
demonstrate various sequence improvements, including: 1)
hexagonal gradient spoiling that minimises ghosting,
shortens TR and reduces confounding T2 sensitivity; 2) low
readout flip angles combined with symmetric z-spectrum
sampling that better maintains the steady state between
samples and eliminates the need for T1-restoration periods;
and 3) exchange-rate matched 360° CEST pulses that reduce
direct water saturation to minimise T1 sensitivity and
increase CNR. Together, these improvements result in
high-quality whole-brain 39-offset z-spectra measurements at
3mm isotropic resolution in 2:59 minutes.
|
| |
10:36
|
0300.
 |
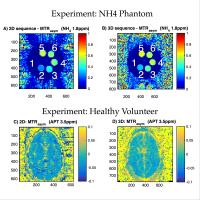
Conjoint measure of 3D ASL and 3D APT in the lesion proximal
regions for differentiating metastasis tumor from glioblastom 
Rui Li1, Bing Wu2, Chien-yuan Lin3,
Xin Lou1, YuLin Wang1, and Lin Ma1
1Department of Radiology, PLA general Hospital,
Beijing, China, People's Republic of, 2GE
healthcare MR Research China, Beijing, China, People's
Republic of, 3GE
heathcare Taiwan, Taipei, Taiwan
Differential diagnosis is challenging due to similar
appearance using conventional imaging such as T1 contrast
enhanced and DWI. In this work, we use the measure of
spatially matching 3D arterial spin labeling (ASL) and 3D
amide proton transfer (APT) in the lesion proximal regions
to differentiate metastasis and glioblastom, in the
hypothesis that glioblastom infiltrates into sourrounding
tissues whereas metastasis tumors have clear biological
boundaries.
|
| |
10:48
 |
0301.
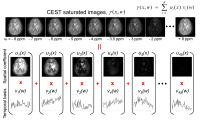 |
Blind Compressed Sensing-based Ultrafast Chemical Exchange
Saturation Transfer (CEST) Imaging 
Hye-Young Heo1,2, Sampada Bhave3,
Mathews Jacob3, and Jinyuan Zhou1,2
1The Russell H. Morgan Department of Radiology
and Radiological Science, Johns Hopkins University School of
Medicine, Baltimore, MD, United States, 2F.M.
Kirby Research Center for Functional Brain Imaging, Kennedy
Krieger Institute, Baltimore, MD, United States, 3Department
of Electrical and Computer Engineering, University of Iowa,
Iowa City, IA, United States
CEST imaging, such as amide proton transfer (APT) imaging,
is a novel, clinically valuable molecular MRI technique that
can give contrast due to a change in water signal caused by
chemical exchange with saturated solute protons. However,
its clinical translation is still limited by its relatively
long scan time because a series of RF saturation frequencies
are unavoidably acquired. Here, we present a highly
accelerated CEST imaging technique (up to 10-fold) using a
novel blind compressed sensing framework.
|
| |
11:00
|
0302.
 |
Separation of intracellular and extracellular Z-spectra by
DiffusionCEST 
Kevin Ray1, Gogulan Karunanithy2,
Andrew Baldwin2, Michael Chappell3,
and Nicola Sibson1
1Oxford Institute for Radiation Oncology,
University of Oxford, Oxford, United Kingdom, 2Physical
and Theoretical Chemistry Laboratory, University of Oxford,
Oxford, United Kingdom, 3Institute
of Biomedical Engineering, University of Oxford, Oxford,
United Kingdom
CEST-MRI is an imaging technique which is sensitive to
tissue pH, and has generated pH-weighted images in acute
stroke patients. One key assumption regarding CEST-MRI is
that the signal is predominantly intracellular. This
assumption has implications in the application of CEST-MRI
for pH measurement of tumours, which are generally
associated with extracellular acidosis. This study developed
a novel pulse sequence, combining stimulated echo
acquisition mode diffusion and CEST imaging. Using this
novel pulse sequence, the intracellular and extracellular
contributions to the acquired Z-spectrum were isolated in a
simple cell system and post-mortem mouse brain.
|
| |
11:12
|
0303.
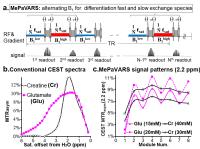 |
Multi-echo Parametric VARiation Saturation (MePaVARS) enabling
more specific endogeneous CEST imaging 
Xiaolei Song1,2, Yan Bai1,3, Meiyun
Wang3, and Michael T. McMahon1,2
1The Russell H. Morgan Department of Radiology
and Radiological Science, Johns Hopkins University School of
Medicine, Baltimore, MD, United States, 2F.M.
Kirby Research Center for Functional Brain Imaging, Kennedy
Krieger Institute, Baltimore, MD, United States, 3Department
of Radiology, Henan Provincial People’s Hospital, Zhengzhou,
China, People's Republic of
Existing CEST methodologies have difficulties in
discriminating agents with small difference in chemical
shift. As CEST signal is very sensitive to saturation power
(B1) and length (tsat), indicating a
second route to indentify agents by modulating the
saturation conditions. We utilized the Multi-echo Parametric
VARiation Saturation (MePaVARS), to separate faster and
slower exchanging endogeneous CEST metabolites and molecules
according to their differences response to B1. In
simulations and phantoms, MePaVARS allowed extraction of
faster-exchanging Glutamate from the slower-exchanging
Creatine, based on its oscillation patterns. A preliminary
study for mice bearing prostate tumor further validated the
feasibility of MePaVARS in
vivo.
|
| |
11:24
|
0304.
 |
Implementing single-shot quantitative CEST/T1? measurements
using bSSFPX 
Shu Zhang1, Robert E Lenkinski1,2, and
Elena Vinogradov1,2
1Radiology, UT Southwestern Medical Center,
Dallas, TX, United States, 2Advanced
Imaging Research Center, UT Southwestern Medical Center,
Dallas, TX, United States
Recently properties of bSSFP were explored to detect
exchange processes (bSSFPX), similar to CEST or
off-resonance T1ρ experiments.
We expand the study and implement a transient bSSFPX
experiment that acquires bSSFP spectra continuously as the
effective saturation time increases, allowing observation of
the approach of magnetization to the steady state in a
single shot. The magnetization dynamic is governed by the
effective field and relaxation times parallel or
perpendicular to it. Work is in progress to derive an exact
quantification model. The method leads to fast acquisition
of time-dependent data and may speed up QUEST-like
quantification of the exchange processes.
|
| |
11:36
 |
0305.
 |
IHMT: Is it misnamed? A simple theoretical description of
"inhomogeneous" MT. 
Alan P Manning1, Kimberley L Chang2,
Alex MacKay1,3, and Carl A Michal1
1Physics and Astronomy, University of British
Columbia, Vancouver, BC, Canada, 2Department
of Neurology, University of British Columbia, Vancouver, BC,
Canada, 3UBC
MRI Research Centre, Department of Radiology, University of
British Columbia, Vancouver, BC, Canada
Inhomogeneous MT (IHMT) shows promise for
myelin-selectivity. Images acquired with soft prepulses at
positive and negative offsets simultaneously show a reduced
intensity compared to images with a single positive or
negative offset prepulse. The leading hypothesis is that
this works due to inhomogeneous broadening of the lipid
proton line. Our results contradict this. We show that IHMT
can be explained by a simple spin-1 model of a coupled
methylene pair, and that it occurs in homogeneously-broadened
systems (hair and wood). We propose the relevant timescales
for IHMT are the dipolar coupling correlation time and the
prepulse nutation period.
|
| |
11:48
|
0306.
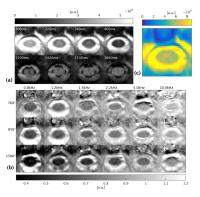 |
In vivo quantitative Magnetisation Transfer in the cervical
spinal cord using reduced Field-of-View imaging: a feasibility
study 
Marco Battiston1, Francesco Grussu1,
James E. M. Fairney2,3, Ferran Prados1,4,
Sebastien Ourselin4, Mara Cercignani5,
Claudia Angela Michela Gandini Wheeler-Kingshott1,6,
and Rebecca S Samson1
1NMR Research Unit, Queen Square MS Centre,
Department of Neuroinflammation, UCL Institute of Neurology,
University College London, London, United Kingdom, 2UCL
Department of Medical Physics and Biomedical Engineering,
University College London, London, United Kingdom, 3Department
of Brain Repair and Rehabilitation, UCL Institute of
Neurology, University College London, London, United
Kingdom,4Translational Imaging Group, Centre for
Medical Image Computing, UCL Department Medical Physics and
Bioengineering, University College London, London, United
Kingdom, 5CISC,
Brighton & Sussex Medical School, Brighton, United Kingdom, 6Brain
Connectivity Center, C. Mondino National Neurological
Institute, Pavia, Italy
Quantitative Magnetization Transfer (qMT) Imaging techniques
offer the possibility to estimate tissue macromolecular
fraction, which has been shown to be specific for myelin in
the brain and spinal cord. To date, applications of qMT in
the spinal cord have been hampered by prohibitive protocol
duration. We propose a novel approach for qMT in the spinal
cord based on the combination of off-resonance saturation
and small field-of-view imaging, with the potential of
reducing the scan time needed to perform qMT in the spinal
cord.
|
|












