| |
16:30
 |
0237.
 |
Association between cortical demyelination and structural
connectomics in early multiple sclerosis 
Gabriel Mangeat1,2, Russell Ouellette2,3,
Constantina Andrada Treaba2,3, Tobias Granberg2,3,
Elena Herranz2,3, Celine Louapre2,3,
Nikola Stikov1,4, Jacob A. Sloane3,5,
Eric C. Klawiter2,3,6, Caterina Mainero2,3,
and Julien Cohen-Adad1,7
1Polytechnique Montreal, Montreal, QC, Canada, 2Athinoula
A. Martinos Center for Biomedical Imaging, MGH, Charlestown,
MA, United States, 3Harvard
Medical School, Boston, MA, United States, 4Montreal
Health Institute, Montreal, QC, Canada, 5Beth
Israel Deaconess Medical Center, Boston, MA, United States, 6Department
of Neurology, MGH, Boston, MA, United States, 7CRIUGM,
Functional Neuroimaging Unit, Universite´ de Montre´al,
Montreal, QC, United States
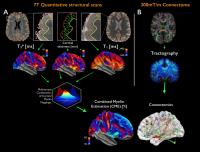
Multiple sclerosis (MS) is a chronic disorder of the central
nervous system characterized by diffuse abnormalities along
white matter tracts and demyelination, including cortical
lesions. In this study, we explored the interplay between
cortical and brain structural networks integrity in a cohort
of early MS subjects by combining quantitative mapping of T2*
and T1 relaxation
rates from 7T MRI acquisitions to measure cortical
demyelination with diffusion imaging and graph theory to
assess the structural brain architecture. Results suggest
that motor, premotor and anterior cingulate cortices are
affected simultaneously by cortical demyelination and
connectomics alterations, at a very early stage of MS.
|
| |
16:42
 |
0238.
 |
Cerebellar-cerebral connections with the default mode network
influence working memory performance in MS 
Giovanni Savini1,2, Matteo Pardini3,
Alessandro Lascialfari1,4, Declan Chard5,
David Miller5, Egidio D'Angelo2,6, and
Claudia Angela Michela Gandini Wheeler-Kingshott2,5
1Department of Physics, University of Milan,
Milan, Italy, 2Brain
Connectivity Center, C. Mondino National Neurological
Institute, Pavia, Italy, 3Department
of Neurosciences, Rehabilitation, Ophthalmology, Genetics
and Maternal and Child Health, University of Genoa, Genoa,
Italy, 4Department
of Physics, University of Pavia, Pavia, Italy, 5NMR
Research Unit, Queen Square MS Centre, Department of
Neuroinflammation, UCL, Institute of Neurology, University
College London, London, United Kingdom, 6Department
of Brain and Behavioral Sciences, University of Pavia, Pavia,
Italy
The cerebellum is linked to the default mode network (DMN)
and its contribution to non-motor functions is now
increasingly recognized. In Multiple Sclerosis (MS) motor
and cognitive functions are both impaired. Here we aimed at
assessing a possible link between cognition and
cerebellar-cerebral fibers disruption in MS. Probabilistic
tractography and graph theory derived metrics were compared
to Symbol Digit Modalities Test (SDMT) scores in MS. We
found that accounting for cerebellar-cerebral connections
when calculating DMN graph metrics yielded a stronger
correlation between network efficiency and SDMT scores,
suggesting that disruption of the cerebellar-cerebral
connections has significant cognitive consequences in MS.
|
| |
16:54
|
0239.
 |
Outer and inner cortical MTR abnormalities observed in
clinically isolated syndromes 
Rebecca Sara Samson1, Manuel Jorge Cardoso2,3,
Wallace J Brownlee1, J William Brown1,4,
Matteo Pardini5, Sebastian Ourselin2,3,
Claudia Angela Michela Gandini Wheeler-Kingshott1,6,
David H Miller1,7, and Declan T Chard1,7
1NMR Research Unit, Queen Square MS Centre,
Department of Neuroinflammation, UCL Institute of Neurology,
University College London, London, United Kingdom, 2Translational
Imaging Group, Centre for Medical Image Computing,
Department of Medical Physics and Bioengineering, University
College London, London, United Kingdom, 3Dementia
Research Centre, Department of Neurodegenerative Diseases,
UCL Institute of Neurology, London, United Kingdom, 4Department
of Clinical Neurosciences, University of Cambridge,
Cambridge, United Kingdom, 5Department
of Neuroscience, Rehabilitation, Ophthalmology, Genetics,
Maternal and Child Health, University of Genoa, Genoa,
Italy, 6Brain
Connectivity Center, C. Mondino National Neurological
Institute, Pavia, Italy, 7National
Institute for Health Research (NIHR) University College
London Hospitals (UCLH) Biomedical Research Centre, London,
United Kingdom
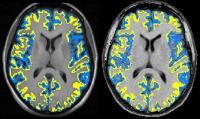
Cortical magnetization transfer ratio (cMTR) is potentially
a sensitive measure of pathology linked with disease
progression in relapse-onset multiple sclerosis (MS). We
investigated outer cMTR changes in people following a
clinically isolated syndrome (CIS), and compared those who
later developed MS with those who did not. Compared with
controls, the outer-to-inner cMTR ratio was significantly
lower in patients who developed MS after 15 years but not in
those who remained CIS. This suggests that the pathological
processes underlying preferential reductions in outer cMTR
start early in the clinical course of MS, and may be
relevant to conversion to MS.
|
| |
17:06
|
0240.
 |
Variable Density Magnetization Transfer (vdMT) imaging for 7 T
MR Imaging 
Se-Hong Oh1, Wanyong Shin1, Jongho Lee2,
and Mark J. Lowe1
1Imaging Institute, Cleveland Clinic Foundation,
Cleveland, OH, United States, 2Laboratory
for Imaging Science and Technology, Department of Electrical
and Computer Engineering, Seoul National University, Seoul,
Korea, Republic of
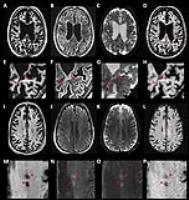
Because of the much higher SAR and longer acquisition time,
in-vivo studies using MT at UHF have not been clinically
feasible. In this work, we demonstrated a new approach
(variable density MT [vdMT])for acquiring whole brain
covered 7T MT data in a clinically reasonable time. vdMT
provides similar image quality to that obtained with
conventional MT imaging, and shortens the scan time by
avoiding from SAR limitation. The proposed method generates
high-resolution MT data in reasonable scan time and it
exhibits high similarity with the conventional method.
Moreover, it maintains sensitivity to MS lesions.
|
| |
17:18
|
0241.
 |
The neuroinflammatory component of gray matter pathology in
multiple sclerosis by in vivo combined 11C-PBR28 MR-PET and 7T
imaging 
Elena Herranz1,2, Costanza Giannì1,2,
Céline Louapre1,2, Constantina Andrada Treaba1,2,
Sindhuja T Govindarajan1, Gabriel Mangeat1,3,
Russell Ouellette1, Marco L Loggia1,2,
Noreen Ward1, Eric C Klawiter1,2,4,
Ciprian Catana1,2, Jacob A Sloane2,5,
Jacob M Hooker1,2, Revere P. Kinkel6,
and Caterina Mainero1,2
1Athinoula A. Martinos Center for Biomedical
Imaging, Department of Radiology, Massachusetts General
Hospital, Boston, MA, United States, 2Harvard
Medical School, Boston, MA, United States, 3Institute
of Biomedical Engineering, Polytechnique Montreal, Montreal,
QC, Canada, 4Department
of Neurology, Massachusetts General Hospital, Boston, MA,
United States, 5Beth
Israel Deaconess Medical Center, Boston, MA, United States, 6University
of California, San Diego, CA, United States
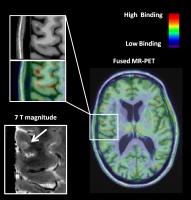
In multiple sclerosis (MS) histopathological investigations
implicated neuroinflammation through microglia and/or
macrophages activation in the pathogenesis of cortical and
subcortical diffuse damage. By combining 11C-PBR28 positron
emission tomography (PET) imaging with anatomical 7T and 3T
MRI, we investigated the presence and correlates of
neuroinflammation in cortex and gray matter of subjects with
MS. We found that neuroinflammation was present in thalamus,
hippocampus, basal ganglia as well as cortex, particularly
cortical lesions, and associated with structural damage,
increased neurological disability and impaired information
processing speed. Our data indicate that neuroinflammation
is closely associated with neurodegeneration.
|
| |
17:30
|
0242.
 |
Quantitative Susceptibility Mapping (QSM) in patients with
clinically isolated syndrome (CIS) and multiple sclerosis (MS) -
a large cohort study 
Ferdinand Schweser1,2, Jesper Hagemeier1,
Paul Polak1, Michael G Dwyer1, Niels P
Bergsland1,3, Nicola Bertolino1,
Bianca Weinstock-Guttman4, Andreas Deistung5,
Jürgen R Reichenbach5,6, and Robert Zivadinov1,2
1Buffalo Neuroimaging Analysis Center, Department
of Neurology, Jacobs School of Medicine and Biomedical
Sciences, The State University of New York at Buffalo,
Buffalo, NY, United States, 2MRI
Molecular and Translational Research Center, Jacobs School
of Medicine and Biomedical Sciences, The State University of
New York at Buffalo, Buffalo, NY, United States, 3MR
Research Laboratory, IRCCS Don Gnocchi Foundation ONLUS,
Milan, Italy, 4Department
of Neurology, Jacobs School of Medicine and Biomedical
Sciences, The State University of New York at Buffalo,
Buffalo, NY, United States, 5Medical
Physics Group, Department of Diagnostic and Interventional
Radiology, Jena University Hospital - Friedrich Schiller
University Jena, Jena, Germany, 6Michael
Stifel Center for Data-driven and Simulation Science Jena,
Friedrich Schiller University Jena, Jena, Germany
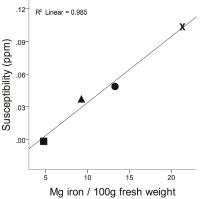
Quantitative susceptibility mapping (QSM) is the most
sensitive technique available for studying tissue iron in
vivo. In this work, we applied QSM to more than 1000
patients with multiple sclerosis (MS) and almost 250
patients with clinically isolated syndrome (CIS). Our
results provide strong support for changed deep gray matter
iron concentrations in MS and CIS.
|
| |
17:42
|
0243.
 |
Dissociated longitudinal patterns of neural activation,
functional connectivity and structural connectivity in a mouse
model of de- and re-myelination 
Yi-Ching Lynn Ho1,2, Fiftarina Puspitasari1,
Way-Cherng Chen1, and Kai-Hsiang Chuang1
1Singapore Bioimaging Consortium, Agency for
Science, Technology & Research (A*STAR), Singapore,
Singapore, 2Interdisciplinary
Institute of Neuroscience & Technology (ZIINT), Zhejiang
University, Hangzhou, China, People's Republic of
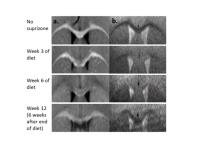
We hypothesized that structure and functional responses do
not demonstrate the same pattern of impairment across time.
Using the cuprizone mouse model of reversible demyelination,
we show different longitudinal patterns of neural activation
and functional connectivity, compared to healthy mice and
also to the extent of cuprizone demyelination.
|
| |
17:54
 |
0244.
 |
Hyperpolarized 13C MRSI can detect neuroinflammation in vivo in
a Multiple Sclerosis murine model 
Caroline Guglielmetti1,2, Chloe Najac1,
Annemie Van der Linden2, Sabrina M Ronen1,
and Myriam M Chaumeil1
1University of California San Francisco, San
Francisco, CA, United States, 2University
of Antwerp, Antwerp, Belgium
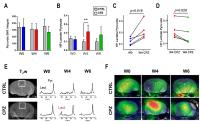
This study demonstrates that 13C
MRS of hyperpolarized pyruvate can be used to detect
increased lactate production from pro-inflammatory
macrophages, mechanism mediated by pyruvate dehydrogenase
kinase-1 upregulation and pyruvate dehydrogenase
inhibition, in a preclinical model of multiple sclerosis,
hence providing a novel tool for in-vivo detection of
neuroinflammation.
|
| |
18:06
 |
0245.
 |
Axon Loss as an Outcome Measure for Assessing Therapeutic
Efficacy 
Tsen-Hsuan Lin1, Mitchell Hallman1,2,
Mattew F. Cusick3, Jane E. Libbey3,
Peng Sun1, Yong Wang1,4,5,6, Robert S.
Fujinami3, and Sheng-Kwei Song1,5,6
1Radiology, Washington University School of
Medicine, St. Louis, MO, United States, 2Perelman
School of Medicine at the University of Pennsylvania,
Philadelphia, PA, United States, 3Pathology,
University of Utah School of Medicine, Salt Lake City, UT,
United States, 4Obstertic
and Gynecology, Washington University School of Medicine,
St. Louis, MO, United States, 5The
Hope Center for Neurological Disorders, Washington
University School of Medicine, St. Louis, MO, United States, 6Biomedical
Engineering, Washington University in St. Louis, St. Louis,
MO, United States
Diffusion basis spectrum imaging (DBSI) has successfully
distinguished co-existing pathologies in CNS, such as MS.
The utility of DBSI derived “axon volume” has not been
explored previously. In this study, we demonstrated the use
of axon loss, reflecting irreversible tissue damage, as an
outcome measure for assessing therapeutic efficacy in a
mouse model of multiple sclerosis.
|
| |
18:18
 |
0246.
 |
In vivo 7T Quantitative Susceptibility Mapping of Cortical
Lesions in Multiple Sclerosis 
Wei Bian1, Eric Tranvinh1, Thomas
Tourdias2, May Han3, Tian Liu4,
Yi Wang4, Brian Rutt1, and Michael
Zeineh1
1Department of Radiology, Stanford University,
Stanford, CA, United States, 2Service
de NeuroImagerie Diagnostique et Thérapeutique, CHU de
Bordeaux, Bordeaux, France, 3Department
of Neurology, Stanford University, Stanford, CA, United
States, 4Department
of Radiology, Weill Medical College of Cornell University,
New York, NY, United States
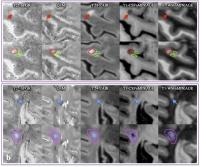
Magnetic susceptibility measured with quantitative
susceptibility mapping (QSM) has been proposed as a
biomarker for inflammation in multiple sclerosis (MS) white
matter (WM) lesions. However, a detailed in vivo
characterization of cortical lesions has not been performed.
In this study, the susceptibility in both cortical and WM
lesions relative to adjacent normal-appearing parenchyma was
measured and compared for 14 MS patients using QSM at 7T.
Our results showed that relative susceptibility was negative
for cortical lesions but positive for WM lesions. The
opposite pattern of relative susceptibility suggests that
iron loss dominates the susceptibility contrast in cortical
lesions.
|
|











