|
|
|
Plasma # |
|
0801.
 |
1 |
Antibody Therapy Against Tau Pathology Improves Neuronal
Transport as Assessed In Vivo by Tract-Tracing
Manganese-Enhanced MRI 
Maria F Baron1, Hameetha Banu Rajamohamed Sait2,
Wajitha J RajaMohamed Sait 2,
D Minh Hoang1, Einar M Sigurdsson2,3,
and Youssef Z Wadghiri1
1Radiology, Center for Advanced Imaging
Innovation & Research (CAI2R) and Bernard and Irene Schwartz
Center for Biomedical Imaging, NYU School of Medicine, New
York, NY, United States,2Neuroscience and
Physiology, NYU School of Medicine, New York, NY, United
States, 3Psychiatry,
NYU School of Medicine, New York, NY, United States
Immunotherapies to target Alzheimer’s pathology have been
developed in recent years. Amyloid-ß centric approaches have
shown limited efficacy, resulting in emphasis on
immunotherapies for clearing pathological tau protein
(τ-Thx). Our group has demonstrated that Tract-Tracing
Manganese Enhanced MRI (TT-MEMRI) is effective to monitor
the deleterious effect of tau pathology on neuronal
transport in transgenic (τ-Tg) mice. In this study, our
TT-MEMRI protocol was used effectively to show the efficacy
of acute tau antibody therapy in an advanced stage of
tauopathy in the Tg model we previously characterized with
TT-MEMRI. Specifically, neuronal transport can be restored
after a four-week treatment period.
|
|
0802.
 |
2 |
In-vivo measurement of a new source of tissue contrast, the
dipolar relaxation time,T1D, using a modified
ihMT sequence 
Gopal Varma1, Valentin H Prevost2,
Olivier M Girard2, Guillaume Duhamel2,
and David C Alsop1
1Radiology, Division of MR Research, Beth Israel
Deaconess Medical Center, Harvard Medical School, Boston,
MA, United States, 2CRMBM-CEMEREM
UMR 7339, CNRS-AMU, Aix Marseille Université, Marseille,
France
The enhanced inhomogeneous magnetization transfer (ihMT) in
certain tissues, especially white matter, has recently been
explained as a result of longer dipolar relaxation times, T1Ds
in those tissues. Measurement of T1D by
modeling the frequency and power dependence of steady state
ihMT has yielded T1D estimates
but with great uncertainty. Here we introduce a dynamic ihMT
experiment that switches between positive and negative
frequency irradiation at varying times. Fits to the ihMT
signal decay curve as a function of switching time at one
(absolute) offset frequency and power enabled highly precise
mapping of T1D that
was largely independent of other MT parameters. A T1D of
6.4±0.5ms for white matter was in good agreement with
reported ex-vivo measurements using Jeener-Broekaert
echoes.
|
|
0803.
 |
3 |
Imaging Reactive Oxygen Species (ROS) using CEST MRI 
Rong-Wen Tain1,2, Alessandro Scotti2,3,
Weiguo Li4,5, Xiaohong Joe Zhou1,2,3,6,
and Kejia Cai1,2,3
1Radiology, College of Medicine, University of
Illinois, Chicago, IL, United States, 23T
Research Program, Center for MR Research, College of
Medicine, University of Illinois, Chicago, IL, United
States,3Bioengineering, College of Engineering,
University of Illinois, Chicago, IL, United States, 4Research
Resource Center, University of Illinois, Chicago, IL, United
States, 5Radiology,
Northwestern University, Chicago, IL, United States, 6Neurosurgery,
College of Medicine, University of Illinois, Chicago, IL,
United States
It is extremely challenging to non-invasively measure tissue
ROS due to its low concentration and short lifetime. This
study aims to demonstrate a fully non-invasive CEST MRI
method to measure ROS concentration. CEST Z-spectra were
acquired from egg white samples with and without hydrogen
peroxide treatment. In addition, proton exchange rate, T1,
and T2 relaxation
time maps were acquired for further clarification on CEST
contrast origin. We have demonstrated that ROS is
paramagnetic and can greatly enhance proton exchange rate
leading to reduced CEST contrast.
|
|
0804.
 |
4 |
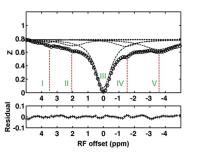
A new NOE-mediated MT signal at -1.6 ppm for detecting ischemic
stroke in rat brain 
Xiaoyong Zhang1,2, Feng Wang1,2,
Aqeela Afzail3, John C. Gore1,2,
Daniel F Gochberg1,2, and Zhongliang Zu1,2
1Vanderbilt University Institute of Imaging
Science, Vanderbilt University, Nashville, TN, United
States, 2Depatment
of Radiology and Radiological Sciences, Vanderbilt
University, Nashville, TN, United States, 3Department
of Neurological Surgery, Vanderbilt University, Nashville,
TN, United States
We recently reported a new NOE-mediated MT signal at around
-1.6 ppm, named NOE(-1.6). In the present work, we evaluated
the changes of this signal that occur early in ischemic
stroke and found that both NOE(-1.6) and Amide Proton
Transfer (APT) signals from stroke lesions have significant
changes after MCAO. Compared with APT, NOE(-1.6) showed much
stronger contrast between stroke and contralateral normal
tissues. We conclude that a new NOE(-1.6) signal in rat
brain could be used as a biomarker for assessment of acute
ischemic stroke.
|
 |
0805.
 |
5 |
3D Amide-Proton-Transfer-Weighted (APTw) Image-Guided
Stereotactic Biopsy in Patients with Newly Diagnosed Gliomas 
Shanshan Jiang1,2, Jaishri Blakeley3,
Charles Eberhart4, Yi Zhang1,
Hye-Young Heo1, Zhibo Wen2, Lindsay
Blair3, Huamin Qin 4,
Michael Lim5, Alfredo Quinones-Hinojosa5,
Dong-Hoon Lee1, Xuna Zhao1, Peter C.M.
van Zijl1, and Jinyuan Zhou1
1Department of Radiology, Johns Hopkins
University, Baltimore, MD, United States, 2Department
of Radiology, Southern Medical University Zhujiang Hospital,
Guangzhou, China, People's Republic of,3Department
of Neurology, Johns Hopkins University, Baltimore, MD,
United States, 4Department
of Pathology, Johns Hopkins University, Baltimore, MD,
United States, 5Department
of Neurosurgery, Johns Hopkins University, Baltimore, MD,
United States
We evaluated the accuracy of the APTw image-guided tissue
biopsy via the neuro-navigation system in newly diagnosed
gliomas. Patients (n = 24) with suspected gliomas of varying
grades were recruited and scanned. APTw image-guided needle
biopsy samples were obtained and analyzed histologically.
Results showed that the APTw signal intensities were
significantly higher in high-grade gliomas than in low-grade
gliomas and that APTw signal intensities had a strong
positive correlation with pathologic cellularity and
proliferation. APTw image-guided biopsy in newly diagnosed
gliomas has the potential to reduce the randomness of
surgical decisions due to tumor heterogeneity.
|
|
0806.
 |
6 |
Magnetic resonance imaging biomarkers for assessment of vascular
pathologies in gliomas 
Andreas Stadlbauer1, Max Zimmermann1,
Karl Rössler1, Stefan Oberndorfer2,
Arnd Dörfler3, Michael Buchfelder1,
and Gertraud Heinz4
1Department of Neurosurgery, University of
Erlangen, Erlangen, Germany, 2Department
of Neurology, University Clinic of St. Pölten, St. Pölten,
Austria, 3Department
of Neuroradiology, University of Erlangen, Erlangen,
Germany, 4Department
of Radiology, University Clinic of St. Pölten, St. Pölten,
Austria
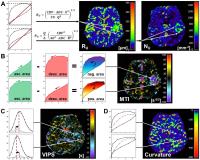
Knowledge about the tumor microvasculature is important for
monitoring of disease progression and treatment response.
Forty-six patients with known or suspected brain tumors were
examined using the vascular architecture mapping (VAM)
technique. ΔR2,GE versus
(ΔR2,SE)3/2 diagrams
were evaluated with new versions of microvessel radius (RU)
and density (NU), which showed increased levels
of heterogeneous structures in glioblastoma and meningioma.
Three new imaging biomarkers were introduced: Microvessel
type indicator (MTI), which allowed differentiation between
supplying arterial and draining venous microvasculature.
Vascular induced peak shift (VIPS), which is more sensitive
to early angiogenic activity. Curvature was increased in
peritumoral vasogenic edema.
|
 |
0807.
 |
7 |
Multi-parametric estimation of brain hemodynamics with
Fingerprinting ASL 
Pan Su1,2, Deng Mao1,2, Peiying Liu1,
Yang Li1,2, Ye Qiao1, and Hanzhang Lu1
1Russell H. Morgan Department of Radiology and
Radiological Science, Johns Hopkins University, Baltimore,
MD, United States, 2Graduate
School of Biomedical Sciences, The University of Texas
Southwestern Medical Center, Dallas, TX, United States
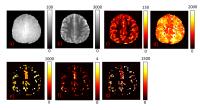
MR Fingerprinting (MRF) based Arterial Spin Labeling (ASL)
has the ability to estimate multiple physiological
parameters in a single scan. In this study, we explored the
potential of this technique by fitting the data to a
three-compartment model to get seven hemodynamic parameters
concomitantly. Hypercapnia study in healthy subjects and
clinical scan in stroke patients were conducted to test
these estimations. Results show that this technique is able
to provide multi-parametric estimations of hemodynamic
markers in healthy and diseased brain.
|
 |
0808.
 |
8 |
Transit time mapping in the mouse brain using time-encoded pCASL 
Lydiane Hirschler1,2,3, Leon P. Munting4,5,
Wouter M. Teeuwisse4, Ernst Suidgeest4,
Jan M. Warnking1,2, Matthias J. P. van Osch4,
Emmanuel L. Barbier1,2, and Louise van der Weerd4,5
1Grenoble Institut des Neurosciences, Université
Grenoble Alpes, Grenoble, France, 2Inserm
U836, Grenoble, France, 3Bruker
Biospin, Ettlingen, Germany, 4Radiology,
Leiden University Medical Center, Leiden, Netherlands, 5Human
Genetics, Leiden University Medical Center, Leiden,
Netherlands
Arterial transit time (ATT) is known to influence
CBF-quantification and is interesting in itself, as it may
reflect underlying vascular pathologies. Currently, no MRI
sequence exists to measure ATT in mice. Recently,
time-encoded labeling schemes have been implemented in rats
and men, enabling ATT-mapping with higher SNR and less
scan-time than multi-delay ASL. In this study, we show that
time-encoded pCASL (te-pCASL) enables transit times
measurements in mice. Furthermore, ATT was found to be
preserved in old WT mice.
|
|
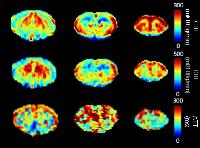
0809.
 |
9 |
Measuring Subtle Leakage in Patients with Cerebrovascular
Disease Using Dual Temporal Resolution DCE-MRI: Is it
Reproducible? 
Sau May Wong1, Jacobus F.A. Jansen1,
C. Eleana Zhang2, Julie Staals2, Paul
A.M. Hofman1, Joachim E. Wildberger1,
Robert J. van Oostenbrugge2, Cécile R.L.P.N.
Jeukens1, and Walter H. Backes1
1Radiology & Nuclear Medicine, Maastricht
University Medical Centre, Maastricht, Netherlands, 2Neurology,
Maastricht University Medical Centre, Maastricht,
Netherlands
Measuring subtle leakage through the blood-brain barrier
using DCE-MRI is challenging since their magnitude is lower
than in high-grade tumors. To have a clinical application,
this method has to be reproducible. The reproducibility of
the transfer constant (Ki) and
fractional plasma volume (vp) using dual
temporal resolution DCE-MRI was investigated in 14 patients
with cerebrovascular disease. Low CVs and moderate to high
ICCs demonstrate that despite the noisy nature of the
measurement, the method is moderately reproducible. Still,
cautious interpretation of the Ki and vp in
individual patients is needed. Day-to-day variations may be
partly compensated by using session-averaged VIFs.
|
 |
0810.
 |
10 |
Modeling demyelination in white matter: the effect of realistic
geometries on the susceptibility-weighted MR signal. 
Tianyou Xu1, Way Cherng Chen2, Michiel
Kleinnijenhuis1, Sean Foxley1, and
Karla L Miller1
1University of Oxford, Oxford, United Kingdom, 2Singapore
Bioimaging Consortium, Singapore, Singapore
Biophysical modeling of axons has conventionally assumed
cylindrical geometries. In reality, axons vary in shape.
Models consisting of circles benefit from simplicity,
however the consequences of this assumption have not been
studied. In this work, simulations incorporating realistic
myelin shape derived from electron microscopy are employed
to model white matter demyelination. Simulations are
compared to a cohort of mice with varying levels of
demyelination. Predictions from models that incorporate
realistic myelin shape are in better agreement with
experimental results in a mouse model of demyelination than
those from circular models.
|
|
0811.
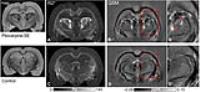 |
11 |
Thalamic nuclei-specific deposits of iron and calcium in the
epileptogenic rat brain revealed by quantitative susceptibility
mapping 
Manisha Aggarwal1, Xu Li2, Peter C van
Zijl2, Olli Gröhn3, and Alejandra
Sierra3
1Department of Radiology, Johns Hopkins
University School of Medicine, Baltimore, MD, United States, 2F.
M. Kirby Research Center, Kennedy Krieger Institute,
Baltimore, MD, United States,3Department of
Neurobiology, A. I. Virtanen Institute for Molecular
Sciences, University of Eastern Finland, Kuopio, Finland
We investigate microstructural pathological alterations in
the epileptogenic rat brain using quantitative
susceptibility mapping (QSM). Using the established model of
pilocarpine-induced status epilepticus (SE), we show for the
first time, localized paramagnetic and diamagnetic
alterations in tissue susceptibility in specific
thalamic-nuclei. QSM contrasts in SE and control rats were
further compared with histological Alizarin and Perls’
stainings, which revealed calcium and iron depositions in
areas corresponding to significant (p<0.005) alterations in
magnetic susceptibility detected in the SE brains. Findings
demonstrate the potential of QSM to sensitively detect and
differentiate localized thalamic nuclei-specific iron and
calcium deposits in the epileptogenic brain.
|
|
0812.
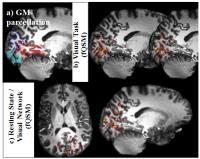 |
12 |
Functional Quantitative Susceptibility Mapping at 7-Tesla:
Resolving Neuronal Activation Localized in Grey-Matter 
Pinar Senay Özbay1,2, Lars Kasper2,
Klaas Paul Pruessmann2, and Daniel Nanz1
1Department of Radiology, University Hospital
Zurich, Zurich, Switzerland, 2Institute
of Biomedical Engineering, ETH Zurich, Zurich, Switzerland
Functional-QSM, promises to offer quantitative information
more directly related to neuronal-activity than BOLD-fMRI
and to partially ameliorate the inherent problem of spatial
mismatch between locations of neuronal-activation and
apparent BOLD-detected-activation. The data for fQSM and
fMRI can be simultaneously acquired and mostly processed
with the well-established fMRI toolchains. The current
high-field study, evaluates details of the processing-chain,
provides clear evidence that fQSM is capable (1) to detect
neuronal-activation in well-resolved volumes that
unambiguously reside within grey-matter, even after removal
of apparent activations associated with larger-veins, and
(2) to identify the visual-network in
resting-state-experiments, thus highlighting a considerable
potential of fQSM.
|
 |
0813.
 |
13 |
Assessing the (ani)sotropic component of R2 as a mean of
studying White Matter properties 
Rita Gil1, Diana Khabipova1,2, Marcel
Zwiers1, Tom Hilbert3,4,5, Tobias
Kober3,4,5, and José P. Marques1
1Donders Institute, Radboud University, Nijmegen,
Netherlands, 2Centre
d'Imagerie BioMédicale, École Polytechnique Fédérale de
Lausanne, Lausanne, Switzerland, 3Advanced
Clinical Imaging Technology (HC CMEA SUI DI BM PI), Siemens
Healthcare AG, Lausanne, Switzerland, 4Department
of Radiology, University Hospital (CHUV), Lausanne,
Switzerland, 5LTS5,
École Polytechnique Fédérale de Lausanne, Lausanne,
Switzerland
In this study we investigate the orientation dependence of
transverse relaxivity (R2) maps in white matter
(WM) due to susceptibility effects of myelin microstructure.
Subjects’ heads were rotated along different orientations
with respect to B0 and
R2 values
(within different WM fibre populations) were decomposed into
R2 isotropic
and anisotropic components (orientation independent and
dependent respectively). Differences found in isotropic
values were associated with fibres different diameter
whereas differences found in anisotropic values were
associated with the susceptibility effects from myelin. It
was showed that the orientation of WM fibres influences R2 contrast
and coherence between hemispheres was also observed.
|
|
0814.
 |
14 |
IN VIVO HYPERCEST DETECTION OF CUCURBIT[6]URIL IN RAT ABDOMEN 
Francis Hane1, Tao Li1, Peter Smylie1,
and Mitchell S Albert1
1Lakehead University, Thunder Bay, ON, Canada
We used the MRI HyperCEST technique to detect the presence
of the xenon encapsulating cage molecule cucurbit[6]uril
(CB6) in the abdomen of a rat. We believe that this is the
first in vivo demonstration of a xenon based biosensor. We
were able to observe a HyperCEST signal depletion of 53%
within the intraperitoneal space of the rat. Our results
demonstrate the feasibility of HyperCEST biosensors to move
from in vitro to in vivo studies.
|
|
0815.
 |
15 |
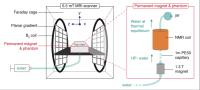
Hyperpolarized saline for contrast-enhanced MR at Ultra-Low
field - Permission Withheld
Najat Salameh1,2,3, Mathieu Sarracanie1,2,3,
Loyd Waites4, David Waddington1,3,5,
and Matthew Rosen1,2,3
1MGH/HST Athinoula A. Martinos Center for
Biomedical Imaging, Charlestown, MA, United States, 2Harvard
Medical School, Boston, MA, United States, 3Department
of Physics, Harvard University, Cambridge, MA, United
States, 4Rensselaer
Polytechnic Institute, Troy, NY, United States, 5ARC
Center for Engineered Quantum Systems, School of Physics,
University of Sydney, Sydney, Australia
Radiologists routinely use contrast-enhanced MRI with
applications mainly in oncology and abdominal imaging. Over
the last decade, researchers have put significant efforts in
developing new probes for molecular imaging where contrast
agents would target only specific cells and/or regions. In
all cases, one main question remains: what is the potential
toxicity of this new contrast agent? We propose here a safe
approach to contrast-enhanced MRI, using pre-polarized
biocompatible saline combined with imaging at ultra-low
field (0.0065 T).
|
|
















