| |
13:30
 |
0685.
 |
Quantitative Susceptibility Mapping for the Evaluation of
Subcortical Iron Abnormality in Parkinson’s Disease with
Dementia 
Darrell Ting Hung Li1, Edward Sai Kam Hui1,
Queenie Chan2, Nailin Yao3, Siew-eng
Chua4, Grainne M. McAlonan4,5, Shu
Leong Ho6, and Henry Ka Fung Mak1
1Department of Diagnostic Radiology, The
University of Hong Kong, Hong Kong, Hong Kong, 2Philips
Healthcare, Hong Kong, Hong Kong, 3Department
of Psychiatry, Yale University, New Haven, CT, United
States,4Department of Psychiatry, The University
of Hong Kong, Hong Kong, Hong Kong, 5Department
of Forensic and Neurodevelopmental Science, King’s College
London, London, United Kingdom, 6Department
of Medicine, The University of Hong Kong, Hong Kong, Hong
Kong
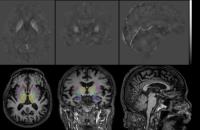
Parkinson’s disease (PD) patients may develop other
non-motor comorbidities when the disease progress. While
increased nigral iron was considered as a biomarker of the
disease, it was also believed that iron deposition is
associated with the development of other non-motor symptoms.
In this study, magnetic susceptibility as a surrogate of
iron concentration was measured in six major subcortical
brain regions on the QSM images. Increased magnetic
susceptibilities were observed in hippocampus and amygdala
of the PD patients with dementia, suggesting a possible
association of iron with the development of dementia symptom
in late stage of PD.
|
| |
13:42
 |
0686.
 |
Mapping temporal order of whole brain volumetric changes using
change point analysis in premanifest Hungtington Disease 
Dan Wu1, Laurent Younes2,3,4, Andreia
V Faria1, Christopher A Ross5, Susumu
Mori1,6, and Michael I Miller3,4,7
1Radiology, Johns Hopkins University School of
Medicine, BALTIMORE, MD, United States, 2Applied
Mathematics and Statistics, Johns Hopkins University,
Baltimore, MD, United States, 3Center
for Imaging Science, Johns Hopkins University, Baltimore,
MD, United States, 4Institute
for Computational Medicine, Johns Hopkins University,
Baltimore, MD, United States, 5Departments
of Psychiatry, Neurology, Neuroscience and Pharmacology, and
Program in Cellular and Molecular Medicine, Johns Hopkins
University School of Medicine, BALTIMORE, MD, United States, 6F.M.
Kirby Research Center for Functional Brain Imaging, Kennedy
Krieger Institute, Baltimore, MD, United States, 7Biomedical
Engineering, Johns Hopkins University, Baltimore, MD, United
States
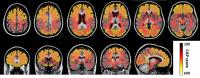
In order to understand the temporal and spatial order of
brain atrophy in Huntington’s disease (HD), we aim to
characterize the whole brain volumetric changes based on
T1-weighted whole brain segmentation. We adapted a novel
multi-variant linear statistical model to capture the change
points of volumetric changing courses from 412 control and
HD subjects. The change point analysis revealed that the
brain atrophy initiated in the deep gray matter structures
and progressed to the peripheral white matter and cortical
regions, and it also suggested the posterior brain atrophy
proceeded the anterior brain.
|
| |
13:54
|
0687.
 |
Connectivity Patterns of Deep Brain Stimulation of the
Subthalamic Nucleus in Parkinson’s Disease 
Silvina G Horovitz1, Nora Vanegas-Arroyave1,2,
Ling Huang2, Peter M Lauro2, Paul A
Taylor3,4,5, Mark Hallett1, Kareem A
Zaghloul6, and Codrin Lungu2
1Human Motor Control Section, National Institute
of Neurological Disorders and Stroke, NIH, Bethesda, MD,
United States, 2Office
of the Clinical Director, National Institute of Neurological
Disorders and Stroke, NIH, Bethesda, MD, United States, 3Scientific
and Statistical Computing Core, National Institutes of
Health, Bethesda, MD, United States, 4Department
of Human Biology, Faculty of Health Sciences, University of
Cape Town, MRC/UCT Medical Imaging Research Unit, Cape Town,
South Africa, 5African
Institute for Mathematical Sciences, Muizenberg, South
Africa, 6Surgical
Neurology Branch, National Institute of Neurological
Disorders and Stroke, NIH, Bethesda, MD, United States
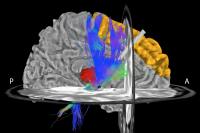
Deep brain stimulation (DBS) of the subthalamic nucleus
(STN) is an effective surgical treatment for Parkinson’s
Disease (PD). However, its mechanism is unclear. We have
developed a pipeline for processing diffusion tensor imaging
(DTI) data in DBS patients, and applied it to analyze 22 PD
patients implanted with bilateral STN-DBS. With this
approach, we have identified the motor nuclei of the
thalamus and the superior frontal cortex as the most common
targets and predictors of clinical benefits.
|
| |
14:06
|
0688.
 |
Simultaneous electrical stimulation of DBS electrodes and fMRI
in movement disorders. 
Stephen Edward Jones1, Hyun-Joo Park2,
Pallab Bhattacharyya1, and Andre Machado2
1Imaging Institute, Cleveland Clinic, Cleveland,
OH, United States, 2Neurologic
Institute, Cleveland Clinic, Cleveland, OH, United States
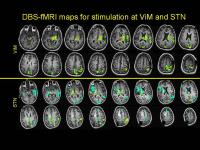
We present a new intra-operative MRI technique for
evaluating placement of DBS electrodes in patients with
movement disorders, using simultaneous electrical
stimulation and fMRI. This technique can elicit a strong
BOLD effect whose pattern can reflect underlying networks.
There is strong spatial sensitivity of these patterns to
electrode position, which is important for clinical utility
in predicting clinical response and unwanted side-effects.
|
| |
14:18
|
0689.
 |
Regional iron accumulation is associated with motor impairments
in Parkinson’s disease as measured by quantitative
susceptibility mapping 
Xiaojun Guan1, Min Xuan1, Quanquan Gu1,
Xiaojun Xu1, Chunlei Liu2,3, Peiyu
Huang1, Nian Wang2, Yong Zhang4,
Wei Luo5, and Minming Zhang1
1Radiology, The Second Affiliated Hospital of
Zhejiang University School of Medicine, Hangzhou, China,
People's Republic of, 2Brain
Imaging and Analysis Center, Duke University School of
Medicine, Durham, NC, American Samoa, 3Department
of Radiology, Duke University School of Medicine, Durham,
American Samoa, 4MR
Research, GE Healthcare, Shanghai, China, People's Republic
of, 5Neurology,
The Second Affiliated Hospital of Zhejiang University School
of Medicine, Hangzhou, China, People's Republic of
We explored the relationships between cerebral iron and the
motor impairments in PD. Quantitative susceptibility
mapping was used to quantify the iron content in vivo.
Iron content in dentate and red nuclei had close
associations with tremor symptom.
Caudate and nigral iron content significantly correlated
with akinetic/rigid symptom.
These might support the idea that regional iron is
related to the motor impairments.
|
| |
14:30
 |
0690.
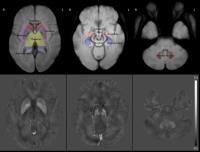 |
Nigral Iron Distribution in Brain of Parkinson’s Disease: A
Combined Structural Voxel-wise and ROI-based Study with
Quantitative Susceptibility Mapping 
Darrell Ting Hung Li1, Edward Sai Kam Hui1,
Queenie Chan2, Nailin Yao3, Siew-eng
Chua4, Grainne M. McAlonan4,5, Shu
Leong Ho6, and Henry Ka Fung Mak1
1Department of Diagnostic Radiology, The
University of Hong Kong, Hong Kong, Hong Kong, 2Philips
Healthcare, Hong Kong, Hong Kong, 3Department
of Psychiatry, Yale University, New Haven, CT, United
States,4Department of Psychiatry, The University
of Hong Kong, Hong Kong, Hong Kong, 5Department
of Forensic and Neurodevelopmental Science, King’s College
London, London, United Kingdom, 6Department
of Medicine, The University of Hong Kong, Hong Kong, Hong
Kong
Abnormal nigral iron deposition is considered one of the
major biomarkers in Parkinson’s disease (PD). Extensive
studies had been performed to evaluate iron concentration in
substantia nigra using different in
vivo imaging
methods. Whole structure ROI-based analysis of basal nuclei
is a majority approach in similar studies. In this study, we
investigated the distribution of iron in substantia nigra
with both voxel-wise and split ROI methods. Location of
significant higher iron concentration was identified to be
around pars compacta of the substantia nigra in PD brain.
The two methods adopted in this study agreed with each
other.
|
| |
14:42
|
0691.
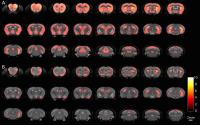 |
Delayed morphological phenotype in R6/2 mice carrying longer
fragments of the human Huntington’s disease gene shown by in
vivo MR imaging and spectroscopy 
Stephen J Sawiak1, Nigel I Wood1, T
Adrian Carpenter1, and A Jennifer Morton1
1University of Cambridge, Cambridge, United
Kingdom
Huntington’s disease is caused by an unstable gene carrying
excessive polyglutamine CAG repeats. Patients with genes
carrying more CAG repeats have a less favourable outcome.
The R6/2 mouse has a fragment of the human HD gene with 100
CAG repeats. We compared mice carrying longer CAG repeats
(250 and 350) with wildtype controls using high-resolution
in vivo longitudinal MRI and spectroscopy. Paradoxically,
the 350CAG mice live longer, with ultimately similar but
much slower atrophy and metabolic changes than 250CAG mice.
They may, therefore, be a more useful model of HD with a
longer window to evaluate pathology and treatments.
|
| |
14:54
|
0692.
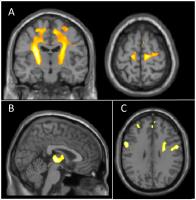 |
Can NODDI provide a better characterisation of microstructural
changes in ALS than DTI? 
Matt Gabel1, Rebecca Broad2, Daniel C.
Alexander3, Hui Zhang3, Nicholas G.
Dowell1, Peter Nigel Leigh2, and Mara
Cercignani1
1Clinical Imaging Sciences Centre, Brighton &
Sussex Medical School, Falmer, United Kingdom, 2Trafford
Centre for Medical Research, Brighton & Sussex Medical
School, Falmer, United Kingdom, 3Centre
for Medical Image Computing, Department of Computer Science,
University College London, London, United Kingdom
NODDI is a multi-compartment model of diffusion MRI that
overcomes some of the limitations of DTI. Our aim was to
assess whether voxelwise analysis of NODDI parameters could
provide a more comprehensive picture than DTI in assessing
the microstructural changes associated with ALS. We analysed
NODDI and DTI parameters for 17 patients with ALS and 19
healthy controls using Advanced Normalization Tools (ANTs)
2.1.0 and SPM12, with age included as a covariate. Both
NODDI and DTI indices are sensitive to pathological changes
in ALS, but NODDI provides more specific tissue
microstructure characterisation.
|
| |
15:06
|
0693.
 |
Cortical Glutathione Deficit in Patients with the “MELAS” A3243G
Mitochondrial DNA Mutation Measured with 1H MRS Documents
Oxidative Stress in the Disorder In Vivo 
Dikoma C. Shungu1, Kristin Engelstadt2,
Xiangling Mao1, Guoxin Kang1, Aya Goji1,
Robert H. Fryer2, Savalatore DiMauro2,
and Darryl C. De Vivo2
1Radiology, Weill Cornell Medical College, New
York, NY, United States, 2Neurology,
College of Physicians and Surgeons of Columbia University,
New York, NY, United States
Although mitochondrial dysfunction has been associated with
redox dysregulation, in
vivo human
brain evidence of such an association is currently lacking.
This study aimed to use 1H
MRS to measure brain levels of the primary tissue
antioxidant glutathione (GSH) in patients with MELAS – a
primary mitochondrial disorder – as an objective marker of
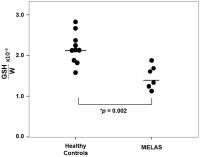
CNS oxidative stress in such disorders. Compared to healthy
control subjects, patients with MELAS showed a 31% lower
cortical GSH levels, thereby directly implicating CNS
oxidative stress as a player in the disorder and pointing to
potential therapeutic interventions based on elevating the
levels of cerebral antioxidants.
|
| |
15:18
|
0694.
 |
CORTICO-SPINAL TRACT AND CEREBELLAR PEDUNCLES PROBABILISTIC
TRACTOGRAPHY IN PARKINSONIAN SYNDROMES 
Stefano Zanigni1,2, Stefania Evangelisti1,2,
Claudia Testa1,2, David Neil Manners1,2,
Giovanna Calandra-Buonaura1,3, Maria Guarino4,
Anna Gabellini3,5, Luisa Sambati1,3,
Pietro Cortelli1,3, Raffaele Lodi1,2,
and Caterina Tonon1,2
1Department of Biomedical and Neuromotor
Sciences, University of Bologna, Bologna, Italy, 2Policlinico
S.Orsola-Malpighi, Functional MR Unit, Bologna, Italy, 3IRCCS
Istituto delle Scienze Neurologiche di Bologna, Bologna,
Italy, 4Policlinico
S.Orsola-Malpighi, Neurology Unit, Bologna, Italy, 5Ospedale
Maggiore, Neurology Unit, Bologna, Italy
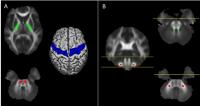
We applied a probabilistic tractography FSL-based method to
evaluate alterations in the cortico-spinal tract (CST),
middle and superior cerebellar peduncles (MCP and SCP,
respectively) in 90 patients with neurodegenerative
parkinsonisms (Progressive Supranuclear Palsy, Multiple
System Atrophy, and Parkinson’s disease). Patients and
healthy controls were evaluated on a 1.5T GE scanner. DTI
metrics were evaluated in the whole CST, MCP and SCP tracts,
and in addition, an along tract analysis for CST has been
performed. We found that specific patterns of
neurodegeneration within these specific tracts are evident
and that they reflect the neuropathological and clinical
profile of each syndrome.
|
|












