Advances in MR Fingerprinting
Advances in MR Fingerprinting
Oral
Oral
Acquisition, Reconstruction & Analysis
Thursday, 16 May 2019
| Room 710B | 08:15 - 10:15 | Moderators: Anthony Christodoulou, Jesse Hamilton |
08:15 |
1100. 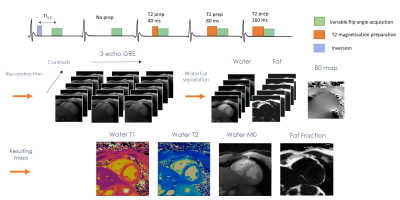 |
Dixon-cMRF: cardiac tissue characterization using three-point Dixon MR fingerprinting
Olivier Jaubert, Gastao Cruz, Aurelien Bustin, Torben Schneider, Rene M. Botnar, Claudia Prieto
Cardiac Magnetic Resonance Fingerprinting (cMRF) has been recently proposed to reduce scan time by estimating simultaneously T1, T2 and M0 in a single breath-hold acquisition. Additionally, cardiac fat images may carry additional diagnostic information and promising results have been shown for epicardial fat volume quantification, characterisation of cardiac masses and detection of fibro-fatty infiltrations. Here we extend cMRF with a three-point Dixon encoding (Dixon-cMRF) for enhanced myocardium tissue characterisation by providing T1, T2, M0 for both water and fat, from which an additional fat fraction map can be computed. The feasibility of Dixon-cMRF was evaluated in 5 healthy subjects.
|
| 08:27 |
1101. 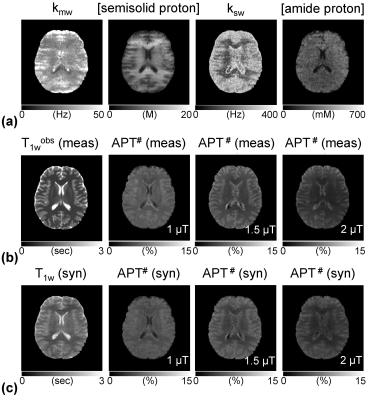 |
Accelerating Quantitative Chemical Exchange Saturation Transfer MRI using MR Fingerprinting and Synthetic CEST Analysis
Hye Young Heo, Zheng Han, Shanshan Jiang, Michael Schar, Peter van Zijl, Jinyuan Zhou
Current CEST-MRI approaches are qualitative in nature, producing contrast that has
|
08:39 |
1102. 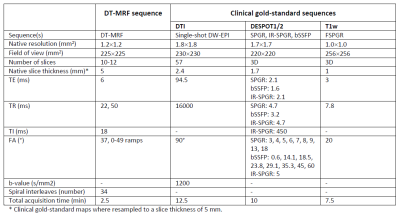 |
Deep Learning-enabled Diffusion Tensor MR Fingerprinting
Carolin Pirkl, Ilona Lipp, Guido Buonincontri, Miguel Molina-Romero, Anjany Sekuboyina, Diana Waldmannstetter, Jonathan Dannenberg, Valentina Tomassini, Michela Tosetti, Derek Jones, Marion Menzel, Bjoern Menze, Pedro Gómez
MR Fingerprinting enables the quantification of multiple tissue properties from a single, time-efficient scan. Here we present a novel Diffusion Tensor MR Fingerprinting acquisition scheme that is simultaneously sensitive to T1, T2 and the full diffusion tensor. We circumvent the long-standing issue of phase errors in diffusion encoding and expensive dictionary matching by using a neural network architecture capable of learning the non-linear relation between fingerprints and multiparametric maps, robustly mitigating motion, undersampling and phase artifacts. As such, our framework enables the simultaneous quantification of relaxation parameters together with the diffusion tensor from a single, highly accelerated acquisition.
|
| 08:51 |
1103. 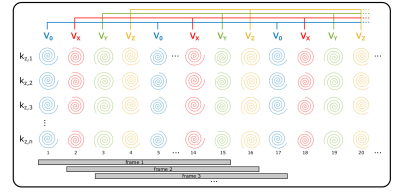 |
Flow-Encoded Magnetic Resonance Fingerprinting: Simultaneous Measurement of Flow, T1, & T2 with Whole Brain Coverage
Sharon Portnoy, Eric Schrauben, Vann Chau, Christopher Macgowan
Incorporating bipolar, flow sensitizing gradients into a magnetic resonance fingerprinting (MRF) pulse sequence permits quantification of vessel flow along with static tissue T1 and T2 relaxation times. This abstract demonstrates quantification of T1, T2 and multi-directional flow with a single, volumetric, flow-encoded MRF acquisition with whole brain coverage. Comparison of T1, T2, and flow measurements with inversion recovery, spin-echo, and phase contrast techniques revealed good agreement between flow-encoded MRF and gold-standard methods. The capacity to obtain volumetric relaxometry and flow measurements with a single acquisition can support efficient, comprehensive assessments of cerebral blood flow and tissue integrity.
|
| 09:03 |
1104. 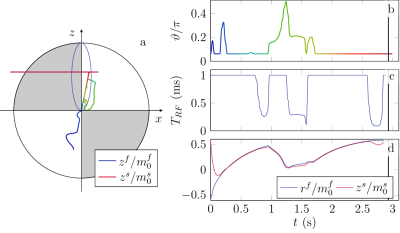 |
Quantitative Magnetization Transfer Imaging in the Hybrid State
Jakob Assländer, Daniel Sodickson
This work extends the hybrid-state model, recently introduced to explain and optimize the dynamics of complex non-steady-state pulse sequences such as MR Fingerprinting, to magnetization transfer. We numerically optimize a hybrid-state pulse sequence for SNR efficiency and demonstrate the potential of the extended hybrid-state framework for quantification of MT effects in vivo in the human brain. Our preliminary results show good agreement with literature and demonstrate the feasibility of quantifying the fractional semi-solid pool size, as well as T1 and T2 relaxation times of the free pool, in under 10 minutes.
|
09:15 |
1105. 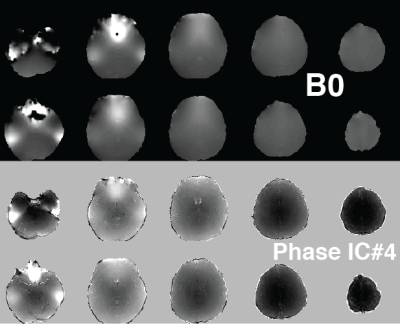 |
Independent Component Analysis of Complex Magnetic Resonance Fingerprinting Data
Rasim Boyacioglu, Debra McGivney, Dan Ma, Mark Griswold
Magnetic Resonance Fingerprinting (MRF) sequences have variable flip angles and TRs that generate unique signal evolutions based on selected tissue properties. To obtain quantitative maps with accurate values in the dictionary matching step it is important to minimize various noise sources or simulate them into the dictionary. We propose to explore systematic artifacts or features that are not in the dictionary with independent component analysis of complex MRF time series. In vivo brain results with 3D MRF revealed global sequence related features (bias from B0 and B1 associated with phase of MRF data) and subject specific reconstruction artifacts.
|
09:27 |
1106. 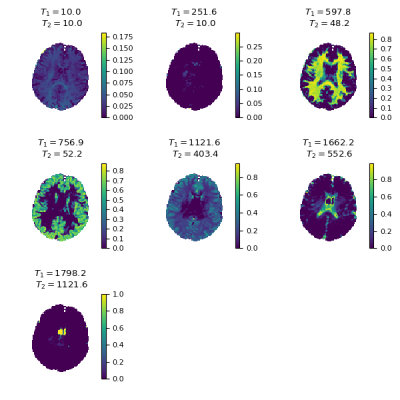 |
MR Fingerprinting multi-component analysis using a fast joint sparsity algorithm
Martijn Nagtegaal, Thomas Amthor, Peter Koken, Mariya Doneva
A new method to perform a multi-component analysis in MR Fingerprinting is proposed, which is faster and less sensitive to noise than previous methods. The algorithm finds a small number of components throughout the region of interest without assumptions about the number or relaxation times of the components. The proposed method is compared to previously published methods in numerical simulations and in vivo experiments.
|
09:39 |
1107. 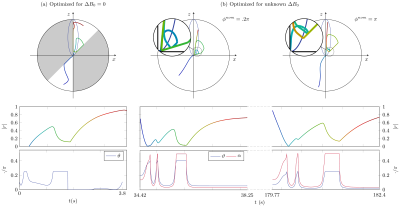 |
Hybrid-State Free Precession for Measuring Magnetic Resonance Relaxation Times in the Presence of B0 Inhomogeneities
Vladimir Kobzar, Carlos Fernandez-Granda, Jakob Asslaender
Magnetic resonance fingerprinting is a methodology for the quantitative estimation of the relaxation times T1,2. An important challenge is to make estimation robust to inhomogeneities of the main magnetic field B0. Free precession sequences with smoothly varying parameters, such as balanced hybrid-state free precession (bHSFP) sequence, can be optimized for T1,2-encoding performance. Previously, magnetic field deviations were assumed to be determined by a separate experiment. Here we develop a numerically optimized bHSFP sequence that takes into account variations in B0 with the aim of mitigating bias due to B0 inhomogeneities. Our numerical results indicate that this approach yields accurate T1,2 estimates when B0 inhomogeneities are unknown.
|
09:51 |
1108. 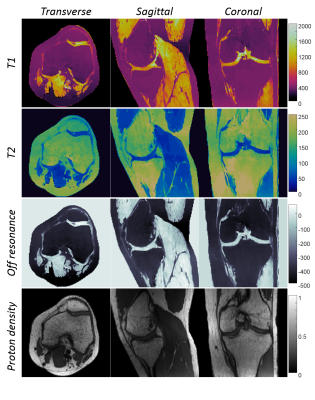 |
Fast 3D MR Fingerprinting with Pseudorandom Cartesian Sampling
Yun Jiang, Gregor Körzdörfer, Jesse Hamilton, Bo Zhao, Lawrence Wald, Nicole Seiberlich, Vikas Gulani, Mathias Nittka, Mark Griswold
A 3D Magnetic Resonance Fingerprinting method based on the pseudorandom Cartesian sampling was proposed to achieve isotropic high-resolution T1, T2 and off-resonance quantification. Compared to existing MRF methods based on non-Cartesian sampling patterns, the proposed method demonstrates a flexible sampling framework that is less susceptible to imperfections of the imaging gradients and off-resonance effects. It provides a step forward to a robust and high-resolution isotropic MRF method in brain, musculoskeletal and abdominal imaging.
|
10:03 |
1109. 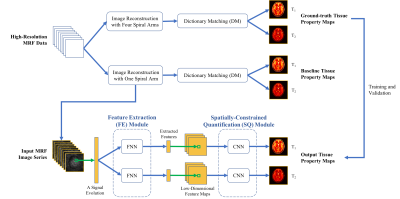 |
Sub-millimeter MR Fingerprinting using deep-learning-based spatially-constrained tissue quantification
Zhenghan Fang, Yong Chen, Weili Lin, Dinggang Shen
MRF is a relatively new quantitative MR imaging technique which can provide rapid and simultaneous quantification of multiple tissue properties. However, high-resolution MRF, particularly at sub-millimeter levels, is technically challenging and often requires extended scan time. In this study, a rapid high-resolution MRF technique was developed using a deep-learning-based spatially-constrained tissue quantification method. The experimental results from in vivo brain data demonstrate that high-quality T1 and T2 quantification with 0.8-mm resolution can be achieved in 15 sec per slice.
|
 Back to Program-at-a-Glance |
Back to Program-at-a-Glance |  Back to Top
Back to Top