Scientific Session
Neurodegeneration 1
| Monday Parallel 2 Live Q&A | Monday, 10 August 2020, 14:30 - 15:15 UTC | Moderators: In-Young Choi |
 |
0211.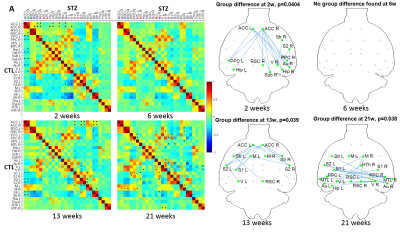 |
Spatio-temporal alterations in functional connectivity, microstructure and cerebral glucose metabolism in a rat model of sporadic Alzheimer’s
Yujian Diao1, Catarina Tristão Pereira2, Carole Poitry-Yamate1, Ting Yin1, Analina Raquel da Silva1, Rolf Gruetter1, and Ileana Ozana Jelescu1
1Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 2Faculdade de Ciências da Universidade de Lisboa, Lisbon, Portugal
Impaired brain glucose consumption is a possible trigger of Alzheimer’s disease (AD). Animal models can help characterize each contributor to the cascade independently. Here we report a comprehensive longitudinal study of functional connectivity, white matter microstructure and brain glucose metabolism using resting-state fMRI, diffusion MRI and FDG-PET in the intracerebroventricular-streptozotocin rat model of AD. Our study highlights the dynamics of how brain insulin resistance affects brain structure and function, and identifies potent MRI-derived biomarkers to track neurodegeneration in human AD and diabetic populations.
|
 |
0212.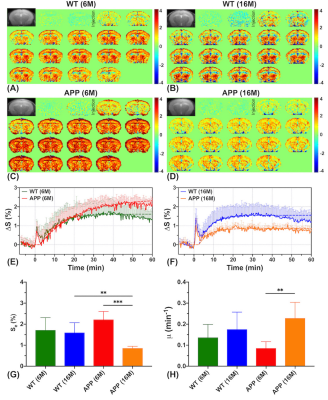 |
Dynamic glucose enhanced (DGE) MRI at 3T detects alterations in glucose uptake and clearance in young and old Alzheimer’s mice
Jianpan Huang1, Xiongqi Han1, Celia M. Dong2, Gerald W. Y. Cheng3, Kai-Hei Tse3, Lin Chen4,5, Joseph H. C. Lai1, Ed X. Wu2, Peter C. M. van Zijl4,5, Jiadi Xu4,5, and Kannie W. Y. Chan1,4
1Department of Biomedical Engineering, City University of Hong Kong, Hong Kong, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China, 3Department of Health Technology and Informatics, The Hong Kong Polytechnic University, Hong Kong, China, 4Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Research Institute, Baltimore, MD, United States
On-resonance variable delay multiple pulse (onVDMP) CEST MRI was applied to detect dynamic D-glucose enhanced signal in brain parenchyma and CSF of 6- and 16-month old APP/PS1 AD mice. A significantly slower D-glucose clearance from CSF was observed in young AD mice compared to age-matched wild type (WT) mice. Moreover, a reduced D-glucose uptake was observed both in parenchyma and CSF of old APP/PS1 mice. D-glucose kinetics detected by onVDMP can be used to assess the alterations in D-glucose uptake and clearance in AD and in the course of AD progression at 3T, a clinical relevant MRI field.
|
 |
0213.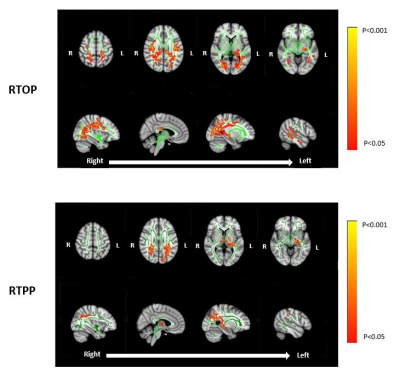 |
Assessing White Matter Microstructural Changes Associated with Mild Cognitive Impairment using Laplacian-Regularized MAP MRI
Jason F. Moody1, Douglas C. Dean III1,2,3, Steven R. Kecskemeti3, Jennifer M. Oh4, Nagesh Adluru3, Sterling C. Johnson4,5, Barbara B. Bendlin4, and Andrew L. Alexander1,2,3,6
1Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Pediatrics, University of Wisconsin-Madison, Madison, WI, United States, 3Waisman Center, University of Wisconsin-Madison, Madison, WI, United States, 4Wisconsin Alzheimer’s Disease Research Center, University of Wisconsin-Madison, Madison, WI, United States, 5Geriatric Research Education and Clinical Center, Middleton Memorial VA Hospital, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Psychiatry, University of Wisconsin-Madison, Madison, WI, United States We implement Laplacian-regularized MAP MRI to investigate distinct white matter (WM) microstructural changes associated with mild cognitive impairment (MCI). Comparisons of diffusion parameters (via TBSS) between healthy controls and MCI patients revealed significant group differences in a wide variety of WM pathways previously shown to be altered in MCI and Alzheimer’s Dementia (AD). In particular, the MCI group exhibited WM clusters with lower return to origin probability (RTOP) and return to plane probability (RTPP) magnitudes, suggesting structurally affected axons in those tracts. Our findings provide an early quantitative framework for identifying specific WM microstructural deficiencies characteristic of MCI and AD. |
 |
0214.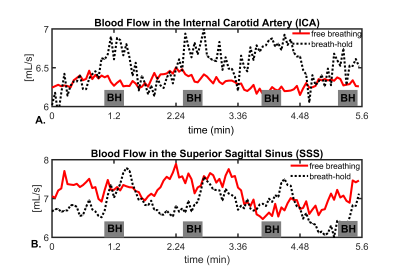 |
Assessment of Intracranial Vascular Flow Oscillations in Alzheimer’s Disease using Real Time 4D Flow MRI
Leonardo A Rivera-Rivera1, Laura B Eisenmenger2, Sterling C Johnson3, and Kevin M Johnson1,2
1Department of Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin - Madison, Madison, WI, United States, 3Department of Medicine, University of Wisconsin - Madison, Madison, WI, United States
Microvascular oscillations have been speculated to be markers of autoregulation and to be driving forces of glymphatic clearance of interstitial fluid, Aβ, and other soluble metabolites of the brain. To probe spontaneous low frequency oscillations (LFO) in the brain vasculature, measures of blood flow variance during several minutes might hold potential. In this study, we investigated induced LFOs in blood flow with 4D flow using 3D radial sampling and low-rank regularization for real time blood flow variance estimates. Preliminary results showed significant increased blood flow fluctuations in age-matched controls compared to AD subjects.
|
 |
0215.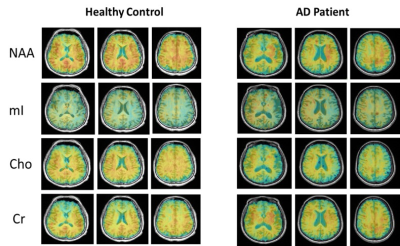 |
Fast 3D High-Resolution Metabolic Imaging in Alzheimer's Disease using SPICE
Jialin Hu1, Miao Zhang2, Rong Guo3,4, Yudu Li3,4, Wanqing Sun1, Danni Wang1, Hui Huang1, Yibo Zhao3,4, Ziyu Meng1,3, Biao Li2, Jun Liu5, Binyin Li5, Jie Luo1, Zhi-Pei Liang3,4,
and Yao Li1
1Institute for Medical Imaging Technology, School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 2Department of Nuclear Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 3Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 4Department of Electrical and Computer Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 5Department of Neurology and Institute of Neurology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
As a progressive neurodegenerative disease, early diagnosis of Alzheimer’s disease (AD) is important but remains difficult. MRSI is a useful tool for detecting neurometabolic alterations in AD, but most studies were limited by using single-slice or single-voxel techniques with low spatial resolution and long data acquisition time. In this study, we performed 3D MRSI of AD patients at a nominal spatial resolution of 2.0 × 3.0 × 3.0 mm3 in a 7-min scan using a new technique called SPICE (SPectroscopic Imaging by exploiting spatiospectral CorrElation). Our experimental results showed noticeable neurometabolic changes in AD patients.
|
 |
0216.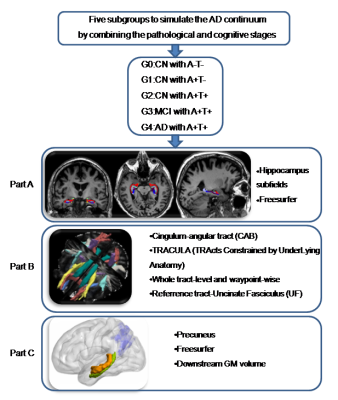 |
Progressive memory circuit impairments along with Alzheimer’s disease neuropathology spread: evidence from in vivo neuroimaging
Shuyue Wang1, Kaicheng Li1, Xiao Luo1, Qingze Zeng1, Yeerfan Jiaerken1, Xiaopei Xu1, Yong Zhang2, Peiyu Huang1, and Minming Zhang1
Video Permission Withheld
1The 2nd Affiliated Hospital of Zhejiang University, School of Medicine, Hangzhou, China, 2GE Healthcare, Shanghai, China
Along with Alzheimer’s disease (AD) continuum, AD neuropathologies propagate trans-neuronally, causing the memory circuit disorganization. The ‘misfolded tau protein propagation theory’ indicates that tau pathology spread through synaptic connectivity and cause the structural impairments. Here, we hypothesized that HP is the first to suffer from AD neuropathology, then followed by the connected tract and downstream cortex. We defined the memory circuit as the hippocampus (HP), cingulum-angular bundles (CAB), and precuneus cortex, respectively representing the starting point, core connecting fibre and connected downstream cortex. Our results support the tau propagation theory in the memory circuit in vivo.
|
 |
0217.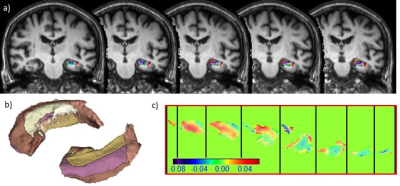 |
Association of Hippocampus Fimbria Iron level measured by QSM with AD stages, Hippocampus Atrophy and Aging
Chun Ki Franklin Au1, Jill Abrigo1, Jack Lee2, Chunlei Liu3, Wing Chi Lisa Au4, Queenie Chan5, Qianyun Maxine Chen1, Chung Tong Vincent Mok4, and Weitian Chen1
1Department of Imaging and Interventional Radiology, The Chinese University of Hong Kong, Hong Kong, Hong Kong, 2Centre For Clinical Research And Biostatistics, Centre for Clinical Research and Biostatistics, The Chinese University of Hong Kong, Hong Kong, Hong Kong, 3Department of Electrical Engineering and Computer Sciences, University of California, Berkeley, USA, CA, United States, 4Division of Neurology, The Chinese University of Hong Kong, Hong Kong, Hong Kong, 5Philips, Hong Kong, Hong Kong
Iron accumulation has been reported in specific brain regions of Alzheimer disease patients. In this study, we used Quantitative Susceptibility Mapping to show iron deposition in hippocampal fimbria and its strong correlation with hippocampus volume and AD stages. Our result might provide further insight of potential disconnection injury in AD pathophysiology.
|
 |
0218.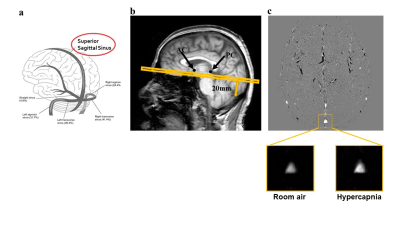 |
Quantitative assessment of cerebrovascular reactivity in older individuals: relationship to diagnosis, cognition and physical function
Sandeepa Sur1, Zixuan Lin1, Yang Li1, Sevil Yasar2, Paul Rosenberg3, Abhay Moghekar4, Shruti Agarwal1, Xirui Hou1, Rita Kalyani5, Kaisha Hazel1, George Pottanat1, Cuimei Xu1, Peter van Zijl6, Jay Pillai7,
Peiying Liu1, Marilyn Albert4, and Hanzhang Lu1
1Department of Radiology, Johns Hopkins University, Baltimore, MD, United States, 2Department of Gerontology, Johns Hopkins University, Baltimore, MD, United States, 3Department of Psychiatry and Behavioral Sciences, Johns Hopkins University, baltimore, MD, United States, 4Department of Neurology, Johns Hopkins University, Baltimore, MD, United States, 5Department of Medicine, Johns Hopkins University, Baltimore, MD, United States, 6F.M. Kirby Research Center, Kennedy Krieger Institute, baltimore, MD, United States, 7Department of Neurosurgery, Johns Hopkins University, Baltimore, MD, United States This study addresses the question, whether quantitative cerebrovascular reactivity (CVR) is a potential vascular biomarker in dementia with Alzheimer’s and vascular pathologies. This was tested in a cross-sectional study, where CBF-CVR assessed via Phase-Contrast-MRI during a CO2 breathing-challenge predicted cognitive and functional performance, disease-severity, and diabetes-risk, in 67 normal and mild-cognitive-impairment subjects. The performance and severity relationships remained robust after adjusting for Alzheimer’s disease and competing vascular markers. These findings suggest that quantitative CBF-CVR has potential as a sensitive biomarker for early changes in cognitive and functional performance, and of disease severity in dementia, independent of Alzheimer’s disease. |
0219.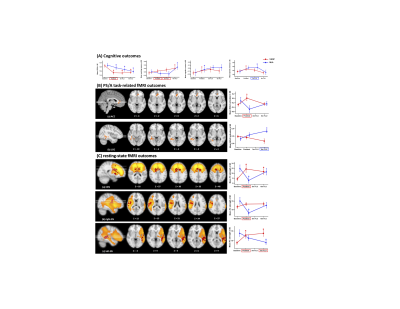 |
The neurocognitive effects of VSOP training in mild cognitive impairment (CogTE study): A phase-II clinical trial
Feng Vankee Lin1
1Center for Advanced Imaging and Neurophysiology, University of Rochester, ROCHESTER, NY, United States
The current lack of effective pharmacological treatments for managing clinical symptoms in Alzheimer’s dementia highlights the urgent need for developing non-pharmacological interventions in the field. Here we report a phase II randomized controlled trial that examined the immediate and mid-term effect of a cognitive process based training on multiple cognitive domains in mild cognitive impairment. We found robust intervention effect on processing speed/attention and working memory. These cognitive improvements were associated with both activation changes and network changes involving ACC, a hub for maintaining successful cognitive aging. These results provide new insights about non-pharmacological interventions in preventing dementia.
|
|
 |
0220.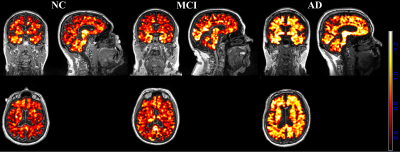 |
Evaluation of neuroinflammation in Alzheimer’s disease on human subjects using third-generation TSPO ligand [18F]-GE180
Zhengshi Yang1, Karthik Sreenivasan1, Xiaowei Zhuang1, Aaron Ritter1, Jessica Caldwell1, Sarah J Banks2, Virendra Mishra1, Marwan Sabbagh1, Dietmar Cordes1,3, and Jeffrey Cummings1,4
1Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV, United States, 2Department of Neuroscience, University of California, San Diego, CA, United States, 3Department of Psychology and Neuroscience, University of Colorado, Boulder, CO, United States, 4Department of Brain Health, School of Integrated Health Sciences, University of Nevada, Las Vegas, NV, United States
Inflammatory reactions contribute to disease progression and severity of Alzheimer’s disease (AD). While multiple animal studies have suggested that increased neuroinflammation occurs in AD, few studies have investigated neuroinflammation in human subjects. This is the first study using the third-generation TSPO ligand [18F]-GE180 to evaluate the neuroinflammation in AD on human subjects. Our study suggests that neuroinflammation accumulates together with amyloid deposition and reaches a plateau when the regional amyloid SUVR reaches 1.1 threshold. Compared to amyloid pathology, neuroinflammation is more closely related to hyperconnectivity in MCI/AD subjects.
|

 Back to Program-at-a-Glance
Back to Program-at-a-Glance Watch the Video
Watch the Video Back to Top
Back to Top