Scientific Session
Neurodegeneration 2
Session Topic: Neurodegeneration 2
Session Sub-Topic: Neurodegeneration
Oral
Neuro
| Wednesday Parallel 2 Live Q&A | Wednesday, 12 August 2020, 15:15 - 16:00 UTC | Moderators: Neil Harris & Yuhei Takado |
Session Number: O-15
 |
0909.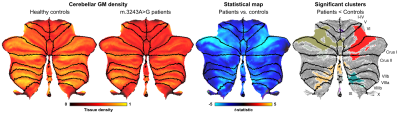 |
Neurodegenerative and functional signatures of the cerebellar cortex in m.3243A>G patients
Roy AM Haast1, Dimo Ivanov2, Ali R Khan1,3, Irenaeus FM de Coo4, Elia Formisano2,5, and Kamil Uludag6,7
1Robarts Research Institute, Western University, London, ON, Canada, 2Department of Cognitive Neuroscience, Maastricht University, Maastricht, Netherlands, 3Department of Medical Biophysics, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada, 4Department of Genetics and Cell Biology, Maastricht University, Maastricht, Netherlands, 5Maastricht Center for Systems Biology, Maastricht University, Maastricht, Netherlands, 6Institute for Basic Science, Center for Neuroscience Imaging Research, Department of Biomedical Engineering, Sungkyunkwan University, Suwon, Republic of Korea, 7University Health Network, Toronto, ON, Canada
The m.3243A>G mutation is the most commonly observed mitochondrial mutation in humans. It causes a wide range of phenotypes, ranging from normal healthy aging to a severely affected quality of life through neuroradiological changes and cognitive impairment. Here, we studied the cerebellar changes in these patients and showed significant local reductions in gray matter tissue volume and functional connectivity using 7T MRI. Interestingly, its white matter remains relatively intact. Taken together, the current results contributes to the still limited understanding of brain pathologies in m.3243A>G patients.
|
0910.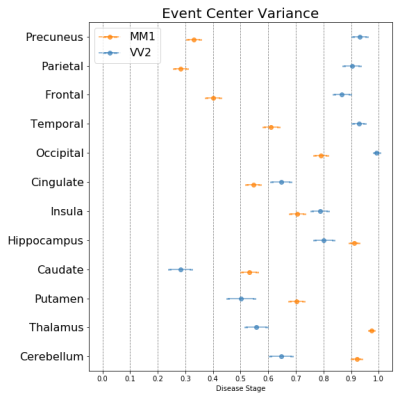 |
Modelling the temporal cascade of abnormalities in diffusion magnetic resonance imaging in sporadic Creutzfeldt-Jakob disease
Riccardo Pascuzzo1, Vikram Venkatraghavan2, Marco Moscatelli1, Marina Grisoli1, Esther E. Bron2, Stefan Klein2, Janis Blevins3, Gianmarco Castelli1, Lawrence B. Schonberger4, Pierluigi Gambetti5, Brian S. Appleby3, and Alberto Bizzi1
1Neuroradiology Unit, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy, 2Biomedical Imaging Group Rotterdam, Departments of Medical Informatics & Radiology, Erasmus MC, University Medical Center Rotterdam, Rotterdam, Netherlands, 3National Prion Disease Pathology Surveillance Center, Case Western Reserve University, School of Medicine, Cleveland, OH, United States, 4National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA, United States, 5Department of Pathology, Case Western Reserve University, School of Medicine, Cleveland, OH, United States
The subtypes of sporadic Creutzfeldt-Jakob disease (sCJD), determined only at autopsy, may have different abnormality patterns in diffusion-weighted magnetic resonance imaging (DW-MRI) according to few reports. For the first time, we provide temporal cascades of the DW-MRI abnormalities in seven distinct sCJD subtypes using a data-driven technique named “discriminative event-based model”. Based on these cascades, we propose a novel procedure to identify the subtype of a patient. We found that sCJD subtypes have either initial cortical (MM/MV1, MM/MV2C, VV1 subtypes) or subcortical involvement (MV2K and VV2) with specific orderings of DW-MRI abnormalities, allowing a correct subtype prediction in most cases.
|
|
 |
0911.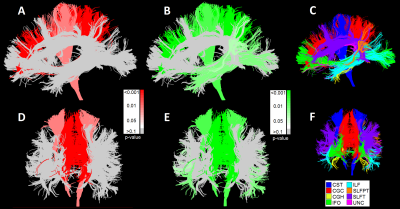 |
Why white matter matters – Interplay of white matter hyperintensities, white matter tracts, and processing speed – The Maastricht Study
Laura W.M. Vergoossen1,2, Jacobus F.A. Jansen1,2,3, Thomas T. van Sloten4,5, Miranda T. Schram2,4,5, Walter H. Backes1,2, and on behalf of The Maastricht Study4
1Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 2Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands, 3Electrical Engineering, Eindhoven University of Technology, Eindhoven, Netherlands, 4Internal Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 5School for Cardiovascular Disease, Maastricht University, Maastricht, Netherlands
White matter hyperintensities interfere with the course of white matter tracts, and disrupt connections between gray matter regions. This process might potentially underlie cognitive decline. In the large population-based Maastricht Study (n=5083), we found an association of lower processing speed scores with larger white matter hyperintensities and smaller total tract volumes in important processing speed related white matter tracts. These findings provide more insight into how white matter hyperintensities seem to influence the cognition-sensitive organization of white matter tracts.
|
 |
0912.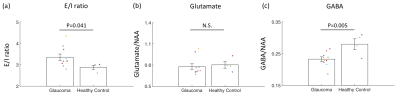 |
Neurochemical alterations in the visual cortex of glaucoma patients
Ji Won Bang1, Anna M Chen1,2, Carlos Parra1, Gadi Wollstein1, Joel S Schuman1, and Kevin Chan1,3
1Department of Ophthalmology, New York University, New York, NY, United States, 2Sackler Institute of Graduate Biomedical Sciences, New York University, New York, NY, United States, 3Department of Radiology, New York University, New York, NY, United States
Glaucoma is considered to involve neurochemical alterations in the visual system. While the role of excitotoxicity in glaucoma remains controversial, we showed that the balance between glutamate, a main excitatory signal, and gamma-aminobutyric acid (GABA), a main inhibitory signal, is involved in glaucoma pathogenesis. We demonstrated that the visual cortex of glaucoma patients changes to an excitatory-dominant state and that this change is driven by reduced GABA. Additionally, we showed that visual field loss is associated with reduced N-acetyl-aspartate, a marker for neuronal integrity. Taken together, these findings suggest that neurochemical alterations may serve as informative markers for glaucoma.
|
 |
0913.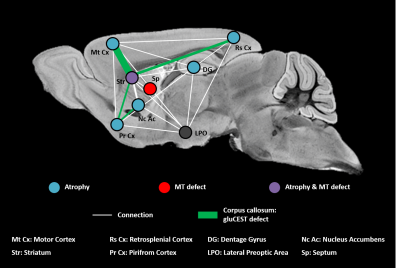 |
Vulnerable brain network in a mouse model of Huntington’s disease revealed by gluCEST, magnetization transfer and anatomic imaging.
Jean-Baptiste Perot1,2, Clement M. Garin1,2, Salma Bougacha1,2,3,4, Alexandra Durr5,6, Marc Dhenain1,2, Sandrine Humbert7, Emmanuel Brouillet1,2, and Julien Flament1,2
1Molecular Imaging Research Center (MIRCen), Commissariat à l'Energie Atomique et aux Energies Alternatives (CEA), Fontenay-aux-Roses, France, 2UMR 9199, Neurodegenerative Diseases Laboratory, Centre National de la Recherche Scientifique (CNRS), Université Paris-Sud, Université Paris-Saclay, Fontenay-aux-Roses, France, 3Inserm UMR-S U1237, Normandie University, UNICAEN, GIP Cyceron, Caen, France, 4Inserm U1077 Neuropsychologie et Imagerie de la mémoire Humaine, Normandie University, UNICAEN, EPHE, CHU de Caen, Caen, France, 5Inserm UMR-S U1127, Institut du Cerveau et de la Moelle épinière (ICM), Sorbonne Université, Paris, France, 6Département de génétique, Groupe Hospitalier Pitié-Salpêtrière, APHP, Paris, France, 7Inserm U1216, Grenoble Institut des Neurosciences (GIN), Univ. Grenoble Alpes, Grenoble, France
Huntington’s disease (HD) is an inherited neurodegenerative disease characterized by cognitive, motor and psychiatric symptoms. Despite tremendous efforts made during past years, there is a need for more predictive and functional biomarkers of disease pathogenesis and progression. In the present study, we developed a longitudinal and multimodal imaging protocol to elucidate HD pathogenesis in a mouse model of HD and to evaluate the potential of different biomarkers. Our approach combining volume, gluCEST and magnetization transfer imaging and automated brain segmentation revealed a brain network particularly vulnerable in this model.
|
 |
0914.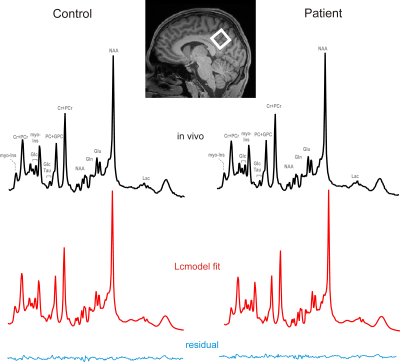 |
Metabolic and microstructural MPSII brain alteration revealed by multiparametric MR imaging and spectroscopy – a combined 3T and 7T study
Alena Svatkova1, Lenka Minarikova2, Petr Bednarik2, Verena Rosenmayr3, Gilbert Hangel2, Bernhard Strasser4, Lukas Hingerl2, Thomas Stulnig3, and Stephan Gruber2
1Department of Medicine III, Medical University of Vienna, Vienna, Austria, 2High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 3Clinical Division for Endocrinology and Metabolism, Department of Medicine III, Medical University of Vienna, Vienna, Austria, 4Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, United States
While glycosaminoglycan deposition in Mucopolysaccharidosis type II, a rare X-linked lysosomal storage disorder, unquestionably alters the brain, metabolic and microstructural MR markers have not been yet established. Thus, we utilized 3T diffusion MRI and fine-tuned semi-LASER MR spectroscopy as well as in-house developed 7T 3D-FID-MRS imaging to examine differences between seven MPSII and eight age-matched healthy males. Analyses revealed profound deficit in the supratentorial white matter consistent with de/dysmyelination on both diffusion and spectroscopy as well as decrease of neuronal population or hypometabolism measured as glutamate deficit in the posterior cingulate cortex, which is a critical hub of neurocognitive networks.
|
0915. |
Neurocognitive and psychiatric features among primary Sjögren’s syndrome patients: from clinical outcomes to brain MRI
Radjiv Goulabchand1,2,3, Veronica Ravano4,5,6, Mário João Fartaria4,5,6, Ricardo Corredor-Jerez4,5,6, Elodie Castille1,3, Sophie Navucet7, Alexandre Maria1,2,8, Alain Le Quellec1,2, Emmanuelle Le Bars9,10,11, Audrey Gabelle2,7,12, Philippe Guilpain1,3,8, Nicolas Menjot de Champfleur9,10, and Bénédicte Maréchal4,5,6
1Département de médecine interne et maladies multi-organiques, Hôpital Saint Eloi, CHRU Montpellier, Montpellier, France, 2Médecine interne, CHU de Nîmes, Nîmes, France, 3Faculté de médecine, Université de Montpellier, Montpellier, France, 4Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 5Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 6LTS 5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 7Centre Mémoire de Ressources et de Recherche, Hôpital Gui De Chauliac, CHRU Montpellier, Montpellier, France, 8IRMB, INSERM, CHU Montpellier, Montpellier, France, 9Département d’imagerie médicale, Hôpital Gui de Chauliac, CHRU Montpellier, Montpellier, France, 10Institut d'Imagerie Fonctionnelle Humaine (I2FH), Hôpital Gui de Chauliac, Centre Hospitalier Régional Universitaire de Montpellier, Montpellier, France, 11Laboratoire Charles Coulomb, CNRS UMR 5221, Université de Montpellier, Montpellier, France, 12Laboratoire de Biochimie-Protéomique Clinique - IRMB - CCBHM - Inserm U1183, CHU Montpellier, Hôpital St-Eloi - Université Montpellier, Montpellier, France
To date, neuropsychiatric profiles in Sjögren’s syndrome patients are not explained by the immunological profile or clinical symptoms. Consequently, there is a lack of biomarkers potentially characterizing such profiles for this rare autoimmune disease. Our goal was to investigate the potential of MRI-based features to objectively explain fatigue, depression and cognitive complaints in twenty-nine patients with primary Sjögren’s syndrome. Specifically, we explored features from automated brain morphometry and brain lesion segmentation as potential imaging biomarkers. Z-score differences in certain brain structures (thalamus, corpus callosum, ventricles, and insula) were found, suggesting an association between MRI-based biomarkers and patient’s neuropsychiatric profiles.
|
|
 |
0916.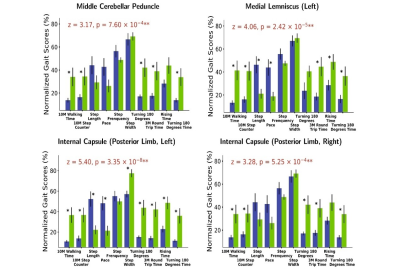 |
Gait - related white matter tracts damage in idiopathic normal pressure hydrocephalus
shuai xu1, ye yao2, jing ding3, and he wang*4,5
1Fudan University, Shanghai, China, 2School of Public Health, Fudan University, Shanghai, China, 3Department of Neurology, Zhongshan Hospital, Fudan University, Shanghai, China, 4Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 5Human Phenome Institute, Fudan University, shanghai, China
Grouping based on white matter hyperactivities (WMH) of each white matter tract, 15 idiopathic normal pressure hydrocephalus (iNPH) patients’ 10 gait index were compared by double sample t test. The results showed some white matter tracts with strongest gait index relationships located in motor and sensory pathways including middle cerebellar peduncle (MCP), left medial lemniscus, left posterior limb of internal capsule and right posterior limb of internal capsule.
|
0917.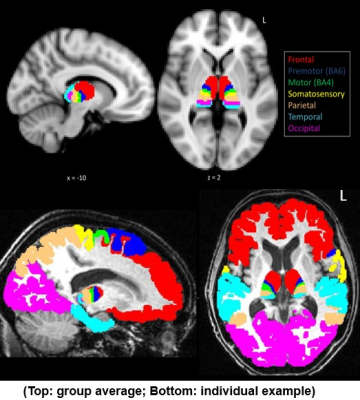 |
Subcortical abnormality reveal disease specific changes in amyotrophic lateral sclerosis
Sicong Tu1,2, Matthew Kiernan1, and Martin Turner2
1Brain and Mind Centre, The University of Sydney, Sydney, Australia, 2Nuffield Department of Clinical Neuroscience, University of Oxford, Oxford, United Kingdom
Amyorophic lateral sclerosis (ALS) is a rapidly progressive neurodegenerative condition affecting the motor system, but increasingly recognised as a multi-system disease. We present two studies that highlight disease specific patterns of abnormality in the thalamus and corpus callosum that suggest regional variation in neural relay structures may be promising markers of disease progression in ALS.
|
|
 |
0918.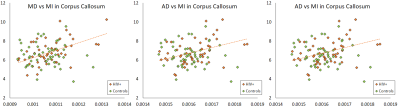 |
A correlation analysis between DTI/DKI derived metrics and metabolite levels in the brain of HIV+ individuals
Teddy Salan1, Sameer Vyas2, Paramjeet Singh2, Mahendra Kumar3, Sulaiman Sheriff1, and Varan Govind1
1Radiology, University of Miami, Miami, FL, United States, 2Postgraduate Institute for Medical Education & Research, Chandigarh, India, 3Psychiatry and Behavioral Sciences, University of Miami, Miami, FL, United States
Several studies have focused on diffusion tensor imaging (DTI) as marker for structural damage in the brain due to infection from human immunodeficiency virus (HIV). However, few have associated the findings from diffusion kurtosis imaging (DKI) and magnetic resonance spectroscopic imaging (MRSI) measures. In this work, we correlate measures of DTI and DKI with MRSI in order to evaluate associations between structural alterations and changes in metabolite concentrations within the brain of HIV individuals.
|

 Back to Program-at-a-Glance
Back to Program-at-a-Glance Watch the Video
Watch the Video Back to Top
Back to Top