Scientific Session
Other Nuclei MRI and MRS to Study Metabolism
Session Topic: Other Nuclei MRI and MRS to Study Metabolism
Session Sub-Topic: X-Nuclei MRS/MRI
Oral
Spectroscopy
| Tuesday Parallel 5 Live Q&A | Tuesday, 11 August 2020, 13:45 - 14:30 UTC | Moderators: Wafaa Zaaraoui & Xiao-Hong Zhu |
Session Number: O-44
0477.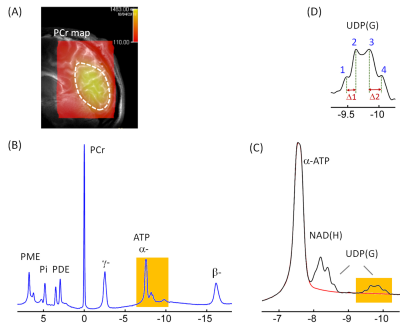 |
Detection of Multiple Nucleotide Sugars Including Uridine Diphosphate Hexoses and N-Acetyl Hexosamines in Human Brain by 31P MRS at 7T
Jimin Ren1,2, Craig R Malloy1,2,3, and A Dean Sherry1,2,4
1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States, 2Department of Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 3VA North Texas Health Care System, Dallas, TX, United States, 4Department of Chemistry, University of Texas at Dallas, Richardson, TX, United States
A variety of nucleotide sugars (NS) are required for glycosylation of proteins and lipids to enhance and diversify cellular functions. The current 7T 31P MRS study, for the first time, reports the detection of four different NS species in human brain in vivo. They are tentatively assigned to UDP-glucose, UDP-galactose, UDP-N-acetylglucosamine, and UDP-N-acetylgalactosamine, collectively denoted as UDP(G). These UDP(G) species are responsible for the observation of a “quartet-like” signal at -9.8 ppm, which cannot be explained by the presence of only a single UDP(G) species such as UDP-glucose (as expected to be a simple doublet).
|
|
 |
0478.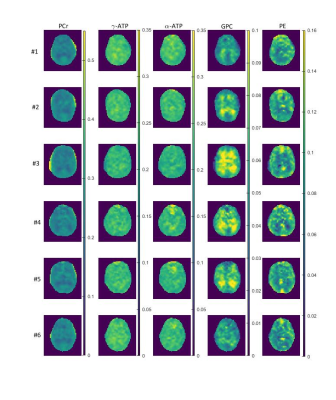 |
Phosphorus metabolic images of the human brain at 9.4 T using Chemical Shift Imaging: Investigation of differences in grey and white matter tissue
Loreen Ruhm1, Johanna Dorst1, Nikolai Avdievich1, and Anke Henning1,2
1Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 2UT Southwestern Medical Center, Dallas, TX, United States
31P Magnetic Resonance Spectroscopic Imaging (MRSI) is a non-invasive method that can reveal information about the energy and phospholipid metabolism. In this work, we investigate the differences in signal amplitudes of different 31P metabolites between grey and white matter tissue in the human brain. We acquired highly resolved 31P MRSI data at an ultrahigh field strength B0 of 9.4 T from the brain of six healthy volunteers. For the quantification of the 31P MRSI data, different correction were applied to the signal amplitudes.
|
0479.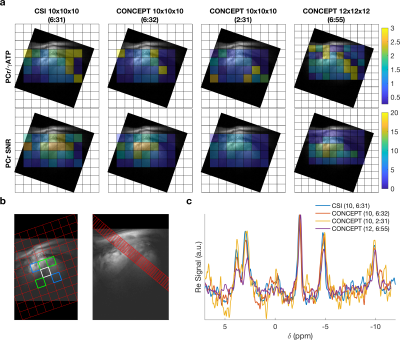 |
31P-MRSI of the human heart in 2 ½ minutes at 7T using concentric rings (CONCEPT)
William T Clarke1, Lukas Hingerl2, Wolfgang Bogner2, Ladislav Valkovic3,4, and Christopher T Rodgers3,5
1Wellcome Centre for Integrative Neuroimaging, NDCN, University of Oxford, Oxford, United Kingdom, 2High-field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 3Oxford Centre for Clinical Magnetic Resonance Research, Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom, 4Department of Imaging Methods, Institute of Measurement Science, Slovak Academy of Sciences, Bratislava, Slovakia, 5Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom
A density-weighted concentric ring trajectory MRSI sequence is implemented for cardiac 31P-MRS at 7T. The sequence is characterised in phantoms and in five healthy participants. Quantitative comparisons are made against a previously implemented acquisition weighted CSI sequence with matched acquisition time and voxel size. The proposed sequence (CONCEPT) was found to robustly measure 3D localised PCr/ATP ratios of the human myocardium 2.59 times faster or with 1.73 times smaller nominal volume than standard acquisition weighted CSI.
|
|
0480.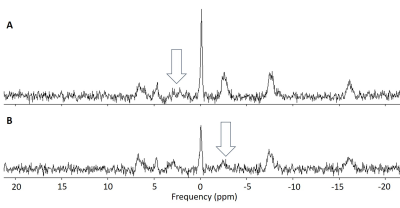 |
Comparison of methods for measuring cerebral mitochondrial function with magnetisation transfer 31P-MRS
Sam Keaveney1, Ross Maxwell1, Maelene Lohézic2, Rolf Schulte3, Ralph Noeske4, and Andrew Blamire1
1Newcastle Magnetic Resonance Centre, Newcastle University, Newcastle upon Tyne, United Kingdom, 2GE Healthcare, Manchester, United Kingdom, 3GE Healthcare, Munich, Germany, 4GE Healthcare, Berlin, Germany
Mitochondrial function in the brain can be measured with magnetisation transfer 31P-MRS. The conventional approach to magnetisation transfer incurs lengthy acquisition times, limiting the available spatial information. An accelerated approach, based on kinetic modelling, allows the technique to be extended to a multi-voxel implementation. Both approaches were applied in a group of healthy subjects to measure the rate of the creatine kinase reaction. There was good agreement between the reaction rates measured with the two methods in equivalently positioned voxels, validating the use of the accelerated approach to provide greater spatial resolution in future patient studies.
|
|
0481.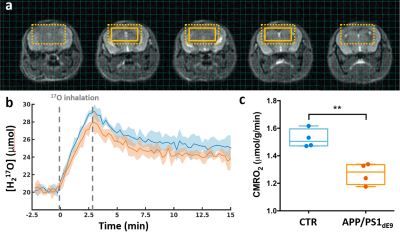 |
Direct 17O-ZTE-MRI reveals decreased cerebral metabolic rate of oxygen consumption in a murine model of amyloidosis
Celine Baligand1,2, Jean-Baptiste Perot1,2, Didier Thenadey1,2, Julien Flament1,2, Marc Dhenain1,2, and Julien Valette1,2
1Molecular Imaging Research Center (MIRCen), Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), Fontenay-aux-Roses, France, 2Neurodegenerative Diseases Laboratory (UMR 9199), Centre National de la Recherche Scientifique (CNRS), Université Paris-Sud, Université Paris-Saclay, Fontenay-aux-Roses, France
The cerebral metabolic rate of oxygen consumption (CMRO2) is an important metric of brain metabolism. It is of particular interest in preclinical studies of Alzheimer’s disease, where amyloidosis has been associated with impaired mitochondrial function. CMRO2 can be measured by direct 17O-MRI of H217O signal changes during inhalation of 17O-labeled oxygen gas. In this study, we used 3D zero echo time 17O-(ZTE-)MRI at 11.7T to measure CMRO2 in the APP/PS1dE9 mouse model of amyloidosis and show that it is significantly lower than in control mice.
|
|
 |
0482.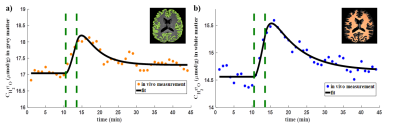 |
Regional Analysis of CMRO2 in Human Brain Using Dynamic 17O-MRI
Hao Song1, Yanis Taege1, Johannes Fischer1, Ali Caglar Özen1,2, and Michael Bock1,2
1Dept. of Radiology, Medical Physics, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2German Consortium for Translational Cancer Research (DKTK) Partner Site Freiburg, German Cancer Research Center (DKFZ), Heidelberg, Germany
In this work, regional quantification of the cerebral metabolic rate of oxygen consumption (CMRO2) was performed using dynamic 17O-MRI with inhalation of isotope-enriched 17O2. First, global mean CMRO2 values were determined in cortical grey matter and white matter. Then, local CMRO2 was investigated in frontal, parietal and occipital areas, respectively. The results were in good agreement with previously reported PET values especially for the cortical grey matter. For regional analysis, the CMRO2 values show good consistency across the white matter and in both frontal and parietal cortical grey matter.
|
 |
0483.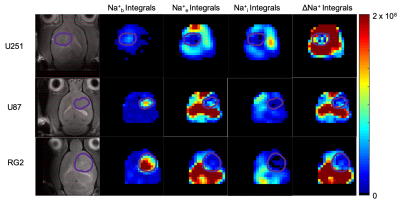 |
Quantitative imaging of the transmembrane sodium gradient in brain tumors
Muhammad H. Khan1, John J. Walsh1, Sandeep K. Mishra2, Daniel Coman2, and Fahmeed Hyder1,2
1Biomedical Engineering, Yale University, New Haven, CT, United States, 2Radiology & Biomedical Imaging, Yale University, New Haven, CT, United States
The transmembrane sodium gradient (difference between extracellular and intracellular concentrations) is necessary for the proper functioning of many bodily mechanisms, including action potential propagation and osmoregulation. While unbound sodium-23 (23Na) is fully detectable by NMR, it is not possible to discriminate between compartments because all 23Na signals resonate at the same frequency. Our objective is to induce 23Na chemical shift differences across cellular compartments with exogenous contrast agents that occupy the interstitial space. We applied this approach successfully in vivo to determine the variation of the transmembrane gradient in glioblastoma.
|
0484.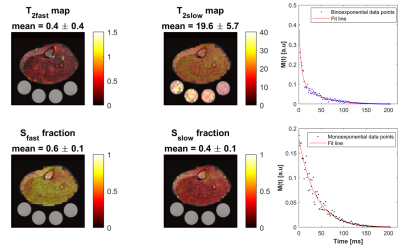 |
Fast In Vivo 23Na Imaging and T2* Mapping Using Non-Localized 2D FID Magnetic Resonance Spectroscopic Imaging at 3 T
Ahmad Alhulail1,2, Pingyu Xia1, Xin Shen3, Miranda Nichols1, Srijyotsna Volety1, Nicholas Farley1, Armin M Nagel4, Ulrike Dydak1,5, and Uzay E Emir1,3
1School of Health Sciences, Purdue University, West Lafayette, IN, United States, 2Department of Radiology and Medical Imaging, Prince Sattam bin Abdulaziz University, Al Kharj, Saudi Arabia, 3Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 4Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 5Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, United States
Sodium signal decays quickly and bi-exponentially, which make T2 relaxation fitting and absolute quantification challenging. Estimating T2 with enough data points using multiple echoes requires an impractical acquisition time. Alternatively, we propose a fast sodium 2D-FID-MRSI sequence to collect the decaying signal with a high sampling frequency (625 Hz) starting at 0.55 ms within only 4 minutes at 3T. We demonstrate an absolute concentration map and separate maps of fast (mean: 0.4 ±0.4 ms) and slow (mean: 19.6 ±5.7 ms) T2* components from human calf muscles, showing that rapid data collection for T2* correction is feasible with this 23Na-MRSI method.
|
|
0485.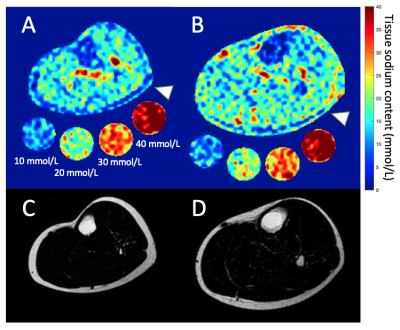 |
Multi-nuclear MRI identifies elevated skin sodium in adults with salt-sensitive blood pressure
Kalen J. Petersen1, Maria Garza1, Cassandra Reynolds2, Deepak Gupta2, Manus J. Donahue1,3,4, and Rachelle Crescenzi1
1Radiology, Vanderbilt University Medical Center, Nashville, TN, United States, 2Cardiology, Vanderbilt University Medical Center, Nashville, TN, United States, 3Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 4Psychiatry, Vanderbilt University Medical Center, Nashville, TN, United States
Salt sensitive blood pressure (SSBP) is a cardiovascular disease risk factor, yet clinically-feasible biomarkers of SSBP have not been developed. We tested the hypothesis that peripheral tissue sodium content (TSC), measured with 23Na-MRI, is higher in persons with vs. without SSBP (n=39 total; age=29.4±7.4 years; sex=21/18 F/M). SSBP was confirmed by independent measurement of BP increase >5 mmHg after high-salt diet compared to low-salt diet. SSBP participants (n=13) had elevated leg skin TSC (p=0.04), and TSC was inversely correlated with leg fat-fraction (ρ=-0.57; p<0.001). Findings suggest that multi-nuclear 23Na/1H-MRI could provide a radiological screening tool for SSBP.
|
|
 |
0486.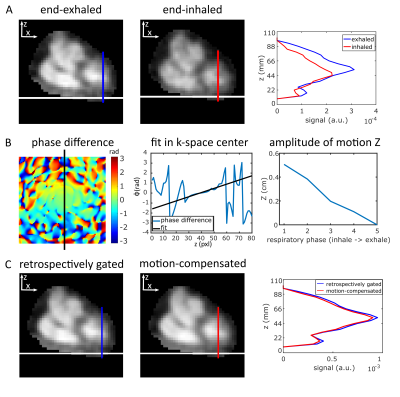 |
Respiratory motion compensation for human cardiac 23Na MRI
Johanna Lott1,2, Armin M. Nagel1,3,4, Sebastian C. Niesporek1, Thoralf Niendorf5,6, Peter Bachert1,2, Mark E. Ladd1,2,7, and Tanja Platt1
1Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, University of Heidelberg, Heidelberg, Germany, 3Institute of Radiology, University Hospital Erlangen, Erlangen, Germany, 4Institute of Medical Physics, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 5Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrueck Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 6MRI. TOOLS GmbH, Berlin, Germany, 7Faculty of Medicine, University of Heidelberg, Heidelberg, Germany
Sodium (23Na) ion distribution plays a fundamental role in biological processes, in particular in myocardial function. 23Na MRI provides noninvasive information about the total tissue sodium concentration. However, short relaxation times, low signal-to-noise ratio, breathing and heart motion render quantitative cardiac 23Na MRI challenging and result in long acquisition times. We present a method to compensate for respiratory motion in 23Na MRI by adding a linear phase in k-space with the goal to determine myocardial tissue sodium concentration. This enables a reduced measurement time for quantitative cardiac 23Na MRI compared to retrospective sorting into one respiratory state.
|

 Back to Program-at-a-Glance
Back to Program-at-a-Glance Watch the Video
Watch the Video Back to Top
Back to Top