Scientific Session
Pediatric Innovations
Session Topic: Pediatric Innovations
Session Sub-Topic: Pediatric High-End Potpourri
Oral
Pediatric
| Monday Parallel 3 Live Q&A | Monday, 10 August 2020, 13:45 - 14:30 UTC | Moderators: Timothy Cain & Dan Wu |
Session Number: O-86
0091.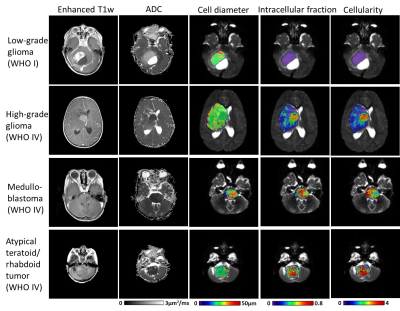 |
Time-dependent Diffusion MRI of Pediatric Brain tumor at 3T
Hongxi Zhang1, Hua Li2, Zhipeng Shen1, Yi Zhang3, and Dan Wu3
1Children's Hospital, Zhejiang University School of Medicine, Hangzhou, China, 2Nemours AI duPont Hospital for Children, Wilmington, DE, United States, 3Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China
Diffusion-time dependent diffusion MRI has shown potential in probing tumor microstructure. This study investigated the feasibility of time-dependent dMRI to map brain tumor microstructure in a pediatric population at 3T. Oscillating and pulsed gradient dMRI was performed to access a series of diffusion times and b-values, and the data were fitted with the IMPULSED model to estimate cell diameter, intracellular fraction, and diffusivity metrics. In a pilot study of 17 pediatric brain tumor patients, all high-grade tumors showed higher intracellular fraction and cellularity than the low-grade ones, while the cell diameter showed differentiation among different types of high-grade tumors.
|
|
 |
0092.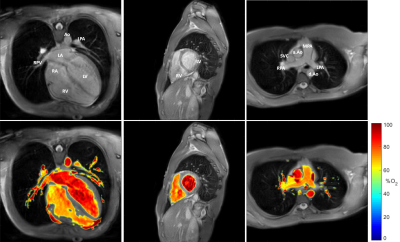 |
Optimized MR blood oximetry using multiple T2 maps: validation with MR-guided catheterization in congenital heart disease
Joshua S. Greer1,2, Daniel A. Castellanos1, Yousef Arar1, Surendranath R. Veeram Reddy1, Yin Xi2, Gerald F. Greil1,2,3, Ananth J. Madhuranthakam2,3, and Tarique Hussain1,2
1Pediatrics, UT Southwestern Medical Center, Dallas, TX, United States, 2Radiology, UT Southwestern Medical Center, Dallas, TX, United States, 3Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States
In this study, a recently proposed MRI blood oximetry technique was optimized to improve the accuracy of oxygen saturation measurements. Simulations of the Luz-Meiboom model were performed to optimize the T2-prep refocusing intervals and to guide the selection of the blood pool used for nuisance parameter estimation. Blood oxygen saturation measurements were validated against MR-guided cardiac catheterization under the same anesthetic conditions in patients with congenital heart disease, with the proposed O2 mapping technique demonstrating improved agreement with blood gas analysis. The proposed improvements may allow for future examination of blood pool oxygenation without the need for invasive cardiac catheterization.
|
 |
0093.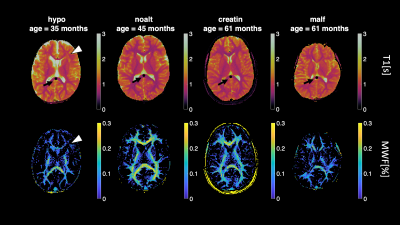 |
Myelin water fraction (MWF) mapping using Magnetic Resonance Fingerprinting (MRF) in a cohort of patients from a child neurology unit
Jan W Kurzawski1,2, Matteo Cencini1,3, Laura Biagi1,4, Graziella Donatelli1,5, Rosa Pasquariello4, Roberta Battini3,4, Claudia Dosi3,4, Chiara Ticci3,4, Alessandra Retico2, Guido Buonincontri1,4, and Michela Tosetti1,4
1Imago7, Pisa, Italy, 2INFN, Pisa, Italy, 3University of Pisa, Pisa, Italy, 4IRCCS Stella Maris, Pisa, Italy, 5Neuroradiology, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy
New advancements in magnetic resonance fingerprinting (MRF) allow more accurate quantification of tissue characteristics and its components using multi-component dictionaries. Recently, a multi-component method for mapping Myelin-Water fraction (MWF) was suggested and validated in healthy children. Here, we studied a cohort with different disorders including hypo- or de- myelinization, Creatine Deficiency Syndrome and brain malformations. We reconstruct MWF maps using tailored dictionaries and estimate fractional myelin values in the splenium of corpus callosum. We observed that myelinization plateaus at around 30 months from birth, while in patients with white matter disorders the process is distorted.
|
 |
0094.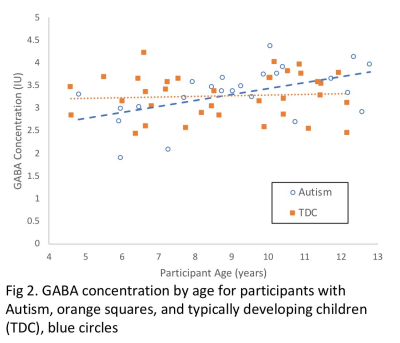 |
Parietal GABA in children with Autism Spectrum Disorder and typically developing peers: distinct age-related changes
Marilena M DeMayo1, Ashley D Harris2,3,4, Ian B Hickie5, and Adam J Guastella1
1Brain and Mind Centre, Children's Hospital Westmead Clinical School, University of Sydney, Camperdown, Australia, 2Department of Radiology, University of Calgary, Calgary, AB, Canada, 3Hotchkiss Brain Institute, Calgary, AB, Canada, 4Alberta Children's Hospital Research Institute, Calgary, AB, Canada, 5Brain and Mind Centre, University of Sydney, Sydney, Australia
GABA, the mature brain’s primary inhibitory neurotransmitter, has been proposed to contribute to the development of Autism Spectrum Disorder (ASD) and the maintenance of ASD symptoms. Investigations have found reductions in GABA in children and adolescents with ASD. In the current study, GABA levels were measured using GABA-edited MEGA-PRESS in the left parietal lobe. The study compared 24 children with ASD and 35 typically developing (TD), aged 4-12 years. Increasing GABA concentration with age was found in the ASD participants but not in the TD cohort, suggesting a distinct pattern of GABA development in ASD within the parietal lobe.
|
0095.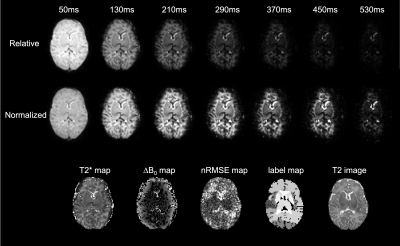 |
Fetal and neonatal whole brain T2* mapping at 3T
Serge Vasylechko1, Emer Hughes2, Joanna Allsop2, Matthew Fox2, Daniel Rueckert1, and Jo Hajnal2
1Biomedical Image Analysis Group, Department of Computing, Imperial College London, London, United Kingdom, 2Centre for the Developing Brain, School of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom
Quantitative T2* mapping in the developing brain is challenging due to inherent motion of fetal and neonatal subjects. This study uses a motion robust framework for acquisition, reconstruction and segmentation of whole brain T2* maps. This is achieved by single-shot multi-echo GRE EPI acquisition, multi-level slice-to-volume registration and gestational-age specific brain atlas segmentation. T2* values are reported for fetal and neonatal subjects at 3T. Findings indicate large variability in T2* within each subject group, non-linear change in T2* between fetal and preterm neonatal period, and significantly higher mean T2* constants than previously reported in adult subjects.
|
|
 |
0096.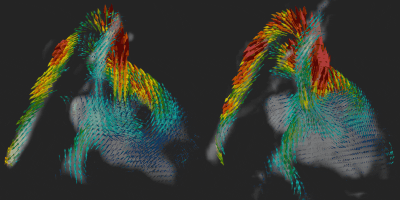 |
Fetal whole-heart 4D blood flow MRI with self-calibrated k-t SENSE
Thomas A Roberts1, Joshua FP van Amerom1,2, Lucilio Cordero-Grande1, Alena Uus1, Anthony N Price1, David FA Lloyd1,3, Laurence H Jackson1, Milou PM van Poppel1, Kuberan Pushparajah3, Mary A Rutherford1, Reza Rezavi1,3, Maria Deprez1, and Joseph V Hajnal1
1School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2Division of Pediatric Cardiology, The Hospital for Sick Children, Toronto, ON, Canada, 3Department of Congenital Heart Disease, Evelina Children's Hospital, London, United Kingdom
Measurement of blood flow in the fetal heart and the great vessels is challenging due to fetal motion and its small size. Previously, we demonstrated use of k-t SENSE real-time 2D imaging combined with slice-to-volume registration to reconstruct 4D velocity cine volumes. This required 50% of the examination to be spent acquiring training data. In this work we combine sliding window reconstruction of the under-sampled target data with some prior knowledge to dispense with training data altogether. We reconstruct 4D blood flow volumes in 5 fetal hearts using both methods and show that they are broadly equivalent.
|
0097.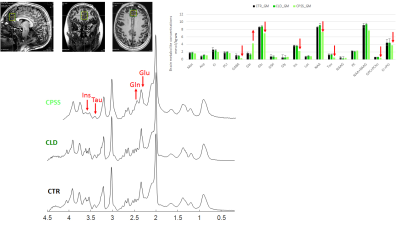 |
Neurometabolism in children with chronic liver disease or portosystemic shunting: a 1H-MRS/MRI study at 7T
Cristina Cudalbu1, Lijing Xin1, Bénédicte Maréchal2,3,4, Tobias Kober2,3,4, Sarah Lachat5, Nathalie Valenza6, Florence Zangas-Gehri6, and Valérie McLin5
1Centre d'Imagerie Biomedicale, Ecole Polytechnique Federale de Lausanne, Lausanne, Switzerland, 2Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 3Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4LTS5, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 5Swiss Pediatric Liver Center, Department of Pediatrics, Gynecology and Obstetrics, University Hospitals Geneva, and University of Geneva Medical School, Geneva, Switzerland, 6Pediatric Neurology Unit, Department of Pediatrics, Gynecology and Obstetrics, University Hospitals Geneva, and University of Geneva Medical School, Geneva, Switzerland
Children with chronic liver disease (CLD) or congenital portosystemic shunts (CPSS) show neurocognitive deficits that are not entirely reversible following liver transplantation or shunt closure. We measured for the first time the neurometabolic profile, brain volumetry and T1 relaxation times of children with CLD and CPSS at 7T. In patients with compensated CLD, there were no significant neurometabolic alterations as assessed by 1H-MRS, while small changes in amygdala and hippocampus volumes were measured. In CPSS, however, neurometabolic changes were pronounced, together with a marked decrease in all measured brain volumes, and likely related to measurably impaired neurocognitive functioning.
|
|
0098.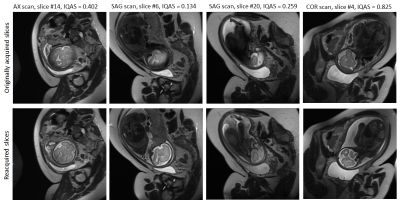 |
Automatic detection and reacquisition of motion degraded images in fetal HASTE imaging at 3T
Borjan Gagoski1,2, Junshen Xu3, Paul Wighton4, Dylan Tisdall5, Robert Frost2,4, Sayeri Lala6, Wei-Ching Lo7, Polina Golland8,9, Andre van der Kouwe2,4, Elfar Adalsteinsson8,10, and P. Ellen Grant1,2
1Fetal Neonatal Neuroimaging and Developmental Science Center, Boston Children's Hospital, Boston, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3(co-first author) Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 5Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 6Department of Electrical Engineering, Princeton University, Princeton, NJ, United States, 7Siemens Medical Solutions USA, Inc, Charlestown, MA, United States, 8Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 9Computer Science and Artificial Intelligence Laboratory (CSAIL), Massachusetts Institute of Technology, Cambridge, MA, United States, 10Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, United States
Fetal brain MRI suffers from unpredictable and unconstrained fetal motion that not only causes severe image artifacts even with single-shot FSE readouts, but also results in slice-to-slice variations of the imaging plane and long scanning sessions, as the MR technologist “chases” the fetal head in an attempt to acquire artifact-free orthogonal images. In this work, we have implemented a closed-loop pipeline that automatically detects and reacquires HASTE images that were degraded by fetal motion, without any interaction from the MRI technologist. The presented methods demonstrate the basic infrastructure needed for successful prospective automated fetal brain motion correction.
|
|
 |
0099.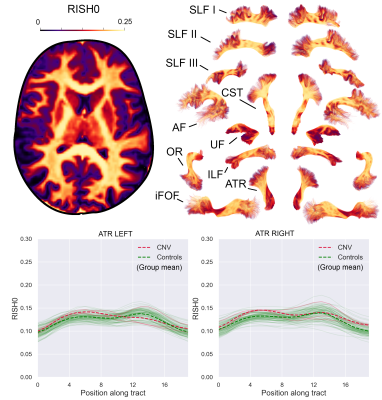 |
Highlighting tract-specific microstructural abnormalities in single subjects using autoencoders
Maxime Chamberland1, Sila Genc1, Erika P Raven1, Chantal M.W. Tax1, Greg D Parker1, Adam Cunningham2, Joanne Doherty1,2, Marianne van den Bree2, and Derek K Jones1
1CUBRIC, Cardiff University, Cardiff, United Kingdom, 2MRC Centre for Neuropsychiatric Genetics and Genomics, Cardiff University, Cardiff, United Kingdom
Most clinical diffusion MRI studies rely on the statistical comparison of a group of patients against a group of healthy controls to make inference about disease. This stymies the potential power of microstructural MRI in the clinic, i.e., to identify microstructural abnormalities in a single patient. We present a framework to address this problem on a case-by-case basis, extending the reach of microstructural imaging to rare cases, where group comparisons are otherwise impossible. Our framework operates on the manifold of white matter pathways and uses autoencoders to learn normative microstructural features, and discriminate patients from controls in a paediatric population.
|
 |
0100.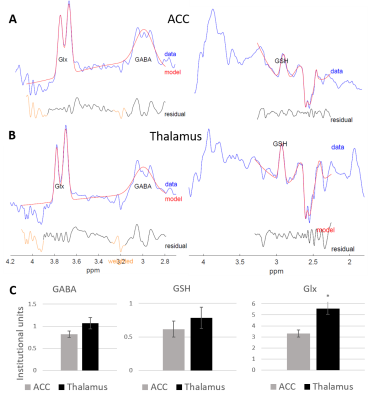 |
Characterising thalamic and anterior cingulate GABA, Glx and GSH in the neonatal brain with HERMES
Maria Yanez Lopez1, Anthony N Price1, Emer Hughes1, Nicolaas AJ Puts2,3, Richard AE Edden2,3, Grainne McAlonan4, Tomoki Arichi1,5, and Enrico De Vita6
1Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins School of Medicine, Baltimore, MD, United States, 3F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 4Department of Forensic and Neurodevelopmental Sciences, Institute of Psychiatry, Psychology & Neuroscience, King's College London, London, United Kingdom, 5Department of Bioengineering, South Kensington Campus, Imperial College London, London, United Kingdom, 6Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
We measured GABA, Glx and GSH levels in a population of healthy neonates, using HERMES at 3T. We show that HERMES can be used to measure significant regional differences (in this case between the thalamus and anterior cingulate cortex). Further application of this method to study how these levels and balance are altered by early-life brain injury or genetic risk can provide important new knowledge about the pathophysiology underlying neurodevelopmental disorders.
|

 Back to Program-at-a-Glance
Back to Program-at-a-Glance Watch the Video
Watch the Video Back to Top
Back to Top