Combined Educational & Scientific Session
Emerging Applications of AI in Neuroimaging
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 6 | 18:00 - 20:00 | Moderators: Seung Hong Choi & Rajan Jain |
 |
Learning from Deep Learning
Eric Oermann
We’ve seen a decade of advances in machine learning and deep learning with a surge of interest in applying these advances to biomedical topics. What are these advances exactly? How are people looking to apply them to biomedical problems and are we finding any answers or only raising more questions? In short, “are we there yet”? I’ll discuss where we are, where we are going, and how I think that we’ll get “there” as Radiology continues to evolve in the coming years.
|
|
 |
Role of AI in Image Acquisition & Processing
Tolga Cukur
MRI offers an unrivaled opportunity to noninvasively examine the structure and function of the human brain. Yet, MRI exams are hindered by limitations on quality and diversity of acquired images due to scan time considerations. Classical approaches to acquisition and processing of imaging data often fail to address these limitations. In this talk, the potential role of machine learning in surpassing these fundamental barriers will be discussed. Novel deep learning techniques for image reconstruction, image synthesis and sampling design will be showcased. State-of-the-art results from these techniques indicate a bright future for machine learning in rapid, high-quality and high-sensitivity neuroimaging.
|
|
 |
AI in Stroke & Hemorrhage Detection
Ona Wu
The use of artificial intelligence and machine learning for stroke research and clinical applications are increasing exponentially every year. Applications in stroke research focus on the extraction of phenotypes that can help in diagnosis, prognosis or management of stroke patients. These applications tend to fall into two major categories – classification or segmentation. We will briefly review some of the more frequently used applications in the domains of acute ischemic stroke and hemorrhage detection. We will also discuss some of the potential pitfalls and ethical questions that may arise.
|
|
 |
AI & NeuroOncology
Spyridon Bakas
This talk introduces computational analytics towards speeding up scientific discovery and personalized diagnostics. It then focuses on two areas; a) radiogenomics, b) data-private collaborations. For radiogenomics, a hypothesis-driven study of the first imaging signature of EGFRvIII in glioblastoma, is presented, followed by data-driven studies on EGFRvIII and other EGFR mutations generating hypothesis for deeper investigations. Then, the first federated learning simulation in healthcare is discussed, followed by the first large-scale real-world federation in medicine, towards developing an AI model to detect glioblastoma boundaries based on 10,000 patients scans from >55 international collaborating sites, without sharing any patient data.
|
| Concurrent 6 | 18:00 - 20:00 | Moderators: |
 |
0659.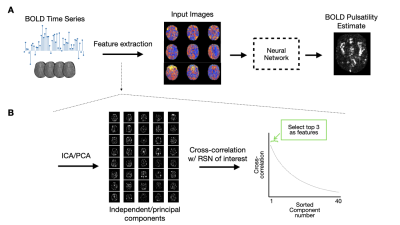 |
A deep learning approach to estimate voxelwise cardiac-related brain pulsatility from BOLD MRI
Nicholas J Luciw1,2, William Cameron2, Andrew D Robertson3, Sarah Atwi2, and Bradley J MacIntosh1,2
1Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Sunnybrook Research Institute, Toronto, ON, Canada, 3Department of Kinesiology, University of Waterloo, Waterloo, ON, Canada
Blood-oxygenation-level-dependent (BOLD) MRI contains both neuronally-mediated and physiological contrast, such as cardiac-related hemodynamic pulsatility. Without a coincident cardiac trace, however, few options exist to assess voxel-specific hemodynamic pulsatility. We investigated the feasibility of training a convolutional neural network to generate accurate cardiac-related pulsatility maps without cardiac trace recordings. Using features derived from the BOLD signal, the network produced pulsatility estimates that were significantly associated with ground truth. This automated method enables investigation of cerebrovascular conditions through the vascular contributions to BOLD data, specifically when cardiac trace recordings are unavailable.
|
|
0660.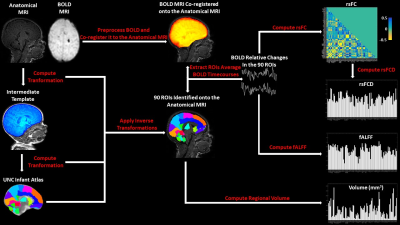 |
Machine Learning Evaluation of the Effects of Prematurity on Regional BOLD Resting-State Activity and Connectivity, and T1-w Brain Volumes.
Antonio Maria Chiarelli1, Carlo Sestieri1, Daniele Mascali1, Richard Geoffrey Wise1, and Massimo Caulo1
1Department of Neuroscience, Imaging and Clinical Sciences, University G. D'Annunzio of Chieti Pescara, Chieti Scalo, Italy
We used Machine Learning (ML) to infer gestational age (GA) at birth, and hence, as a metric of prematurity extent, assess its effect, in 88 premature infants using T2*-w BOLD resting-state connectivity and activity, and T1-w volume in 90 brain regions. ML was able to infer GA at birth. Analysis of the spatial distribution of effects indicated that volumetric alterations, in common with BOLD activity, are partially localized to subcortical structures, but are associated with widespread alterations of connectivity. Our results suggest a potential role for ML in early prediction of neurodevelopmental outcome based on BOLD and anatomical MRI metrics.
|
||
0661.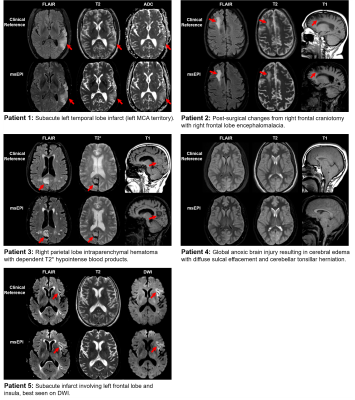 |
Clinical evaluation of an AI-accelerated two-minute multi-shot EPI protocol for comprehensive high-quality brain imaging
Bryan Clifford1, John Conklin2, Susie Huang2, Thorsten Feiweier3, Zahra Hosseini4, Augusto Lio M. Goncalves Filho2, Azadeh Tabari2, Serdest Demir2, Wei-Ching Lo1, Maria Gabriela Figueiro Longo2, Michael Lev2, Pam Schaefer2, Otto Rapalino2,
Kawin Setsompop5,6, Berkin Bilgic7, and Stephen Cauley7
1Siemens Medical Solutions USA, Inc., Boston, MA, United States, 2Department Radiology, Massachusetts General Hospital, Boston, MA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Siemens Medical Solutions USA, Inc., Atlanta, GA, United States, 5Department Radiology, Stanford University, Stanford, CA, United States, 6Department of Electrical Engineering, Stanford University, Stanford, CA, United States, 7Department Radiology, A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
This work integrates a novel machine learning-based reconstruction and optimized magnetization transfer preparation modules with a multi-shot echo-planar imaging acquisition to provide comprehensive whole-brain imaging in two minutes. Neuroradiologist evaluation indicated that the proposed method can produce T2, T2*, T1, FLAIR, and DWI images with SNR, tissue contrast, and lesion conspicuity similar to that of a 10-minute turbo spin echo-based exam. To accommodate a wide range of radiologist preferences and/or hardware configurations without the need for additional training, the proposed method provides a tunable parameter for controlling the level of denoising.
|
||
 |
0662.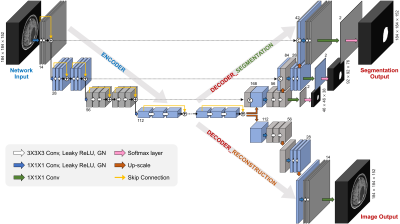 |
Deep Learning-based Automatic Detection and Segmentation of Brain Metastases Using Multi-Task Learning with 3D Black-Blood and GRE Imaging
Yohan Jun*1, Yae Won Park*2, Yangho Lee1, Kyunghwa Han2, Chansik An3, Seung-Koo Lee2, Sung Soo Ahn**2, and Dosik Hwang**1
1Electrical and Electronic Engineering, Yonsei University, Seoul, Korea, Republic of, 2Department of Radiology and Research Institute of Radiological Science and Center for Clinical Imaging Data Science, Yonsei University College of Medicine, Seoul, Korea, Republic of, 3Research and Analysis Team, National Health Insurance Service Ilsan Hospital, Goyang, Korea, Republic of
For the detection of brain metastases, either contrast-enhanced 3D gradient echo (GRE) or spin echo (SE) imaging with black-blood (BB) imaging techniques are commonly used. The objective of this study was to evaluate whether a deep learning (DL) model using both 3D BB imaging and 3D GRE imaging may improve the detection and segmentation performance of brain metastases compared to that using only 3D GRE imaging. We demonstrated that the combined 3D BB and 3D GRE DL model can improve the detection and segmentation performance of brain metastases, especially in detecting small metastases.
|
|
0663.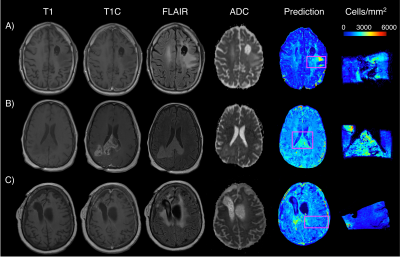 |
Radio-pathomic models trained with autopsy tissue samples aligned to MP-MRI predict histopathological features in brain cancer patients.
Samuel Bobholz1, Allison Lowman2, Michael Brehler2, Savannah Duenweg1, Fitzgerald Kyereme2, Elizabeth Cochran3, Jennifer Connelly4, Wade Mueller5, Mohit Agarwal2, Darren O'Neill2, Anjishnu Banerjee6, and Peter LaViolette2,7
1Biophysics, Medical College of Wisconsin, Milwaukee, WI, United States, 2Radiology, Medical College of Wisconsin, Milwaukee, WI, United States, 3Pathology, Medical College of Wisconsin, Milwaukee, WI, United States, 4Neurology, Medical College of Wisconsin, Milwaukee, WI, United States, 5Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 6Biostatistics, Medical College of Wisconsin, Milwaukee, WI, United States, 7Biomedical Engineering, Medical College of Wisconsin, Milwaukee, WI, United States
This study used autopsy tissue in order to develop radio-pathomic models for histopathological features of brain cancer. These models used T1, T1C, FLAIR, and ADC images from 45 patients as input into bagged regression ensembles for cellularity, cytoplasm, and extracellular fluid, using the aligned autopsy tissue samples as ground truth. These models were able to accurately predict these features and were able to find tumor signatures, such as hypercellularity beyond the traditional contrast-enhancing and FLAIR hyperintense regions. These radio-pathomic maps provide new insights into non-invasive signatures of tumor pathology in the post-treatment state and beyond the contrast enhancing region.
|
||
 |
0664.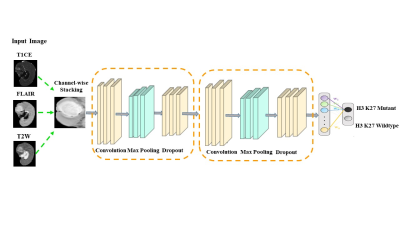 |
Deep learning based prediction of H3K27M mutation in midline gliomas on multimodal MRI
Priyanka Tupe Waghmare1, Piyush Malpure2, Manali Jadhav2, Abhilasha Indoria3, Richa Singh Chauhan4, Subhas Konar5, Vani Santosh3, Jitender Saini6, and Madhura Ingalhalikar7
1E &TC, Symbiosis Institute of Technology, Pune, India, 2Symbiosis Center for Medical Image Analysis, Pune, India, 3National Institute of Mental Health and Neurosciences, Bangalore, India, 4National Institute of Mental Health & Neurosciences, Pune, India, 5National Institute of Mental Health & Neurosciences, Bangalore, India, 6Department of Neuroimaging & Interventional Radiology, National Institute of Mental Health & Neurosciences, Bangalore, India, 7Symbiosis Center for Medical Image Analysis and Symbiosis Institute of Technology, Pune, India
In midline gliomas, patients with H3K27M mutation have poor prognosis and shorter median survival. Moreover, since these tumors are located in deep locations biopsy can be challenging with substantial risk of morbidity. Our work proposes a non-invasive deep learning-based technique on pre-operative multi-modal MRI to detect the H3K27M mutation. Results demonstrate a testing accuracy of 69.76% on 51 patients. Furthermore, the class activation maps illustrate the regions that support the classification. Overall, our preliminary results provide a testimony that multimodal MRI can support identifying H3K27M mutation and with further larger studies can be translated to clinical workflow.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.