Oral
Breast: What's New
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 7 | 12:00 - 14:00 | Moderators: Christopher Comstock & Rebecca Rakow-Penner |
 |
0053.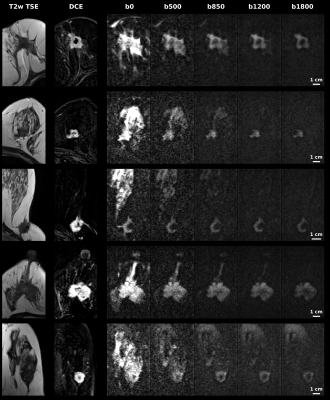 |
Diffusion weighted and kurtosis breast cancer imaging for b=0-1800 s/mm2: Comparisons to dynamic contrast enhanced MRI
Martins Otikovs1, Noam Nissan2, Edna Furman-Haran1, Debbie Anaby2, Tanir M. Allweis3, Ravit Agassi4, Miri Sklair-Levy2,5, and Lucio Frydman1
1Weizmann Institute of Science, Rehovot, Israel, 2Sheba Medical Center, Ramat Gan, Israel, 3Kaplan Medical Center, Rehovot, Israel, 4Ben Gurion University Hospital, Beer Sheba, Israel, 5Tel Aviv University, Tel Aviv, Israel
Spatiotemporal encoding (SPEN) is an alternative ultrafast imaging technique which allows to overcome distortions otherwise observed along EPI’s phase-encoded dimension, and to perform self-referenced phase corrections in interleaved diffusion weighted imaging (DWI) scans. This study compares SPEN’s performance against multishot read-out segmented EPI (RESOLVE), with an emphasis on high-b-valued DWI and diffusion kurtosis imaging in the context of breast cancer imaging. The results show SPEN’s advantages for delivering kurtosis maps that provide a separation between cancerous and healthy tissues. The potential of using SPEN DW images acquired with high b-values as an alternative to DCE subtractions is also assessed.
|
|
 |
0054.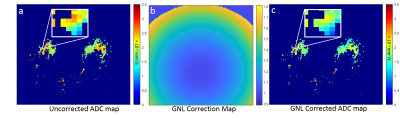 |
Impact of retrospective gradient nonlinearity correction on lesion ADCs and performance in the ECOG-ACRIN A6702 multicenter breast DWI trial
Debosmita Biswas1, Justin Romanoff2, Dariya Malyarenko3, Wesley Surrento4, Habib Rahbar1, Nola Hylton5, David C Newitt5, Thomas L Chenevert3, and Savannah C Partridge1
1Radiology, University of Washington, Seattle, WA, United States, 2Center for Statistical Sciences, Brown University, Providence, RI, United States, 3Radiology, University of Michigan, Ann Arbor, MI, United States, 4Biomedical and Health Informatics, University of Washington, Seattle, WA, United States, 5Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States
Gradient nonlinearity (GNL) correction shows potential to improve the accuracy of ADC values collected across different MRI platforms. Here, we retrospectively applied GNL correction to breast DWI datasets collected in the ECOG-ACRIN A6702 trial by pixel-wise scaling of the ADC map with correction factor map. Our findings confirm that GNL significantly impacts multicenter breast lesion ADC values, and that GNL-based ADC errors vary significantly across MRI vendors and gradient systems. Therefore, GNL correction is important for implementation of generalizable ADC thresholds for separating benign and malignant lesions.
|
|
 |
0055.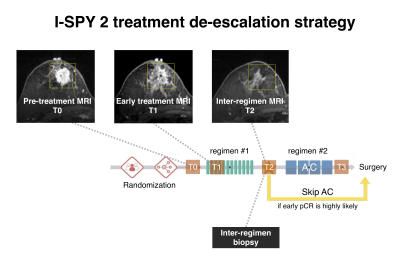 |
Breast MRI functional tumor volume segmentation quality may impact the prediction of pathological complete response
Natsuko Onishi1, Jessica Gibbs1, Teffany Joy Bareng1, Wen Li1, Elissa R. Price1, Bonnie N. Joe1, Laura J. Esserman2, The I-SPY 2 Consortium3, David C. Newitt1, and Nola M. Hylton1
1Department of Radiology & Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Department of Surgery, University of California, San Francisco, San Francisco, CA, United States, 3Quantum Leap Healthcare Collaborative, San Francisco, CA, United States
In the I-SPY2 neoadjuvant breast cancer trial, functional tumor volume (FTV) derived from dynamic contrast-enhanced MRI serves as a key marker. Participants in I-SPY2 have the option to “de-escalate” therapy if achievement of pathological complete response (pCR) is highly likely at inter-regimen time point. A model combining FTV-based predictive probabilities with inter-regimen core-biopsy pathology is central to select candidates for this option. This retrospective study compared the performance of longitudinal FTVs in predicting pCR between optimal and non-optimal FTV segmentation groups. The results suggest that improvements to FTV segmentation can improve FTV’s ability to provide predictive guidance for treatment de-escalation.
|
|
 |
0056.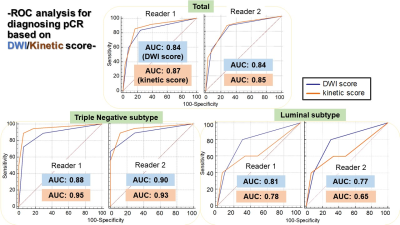 |
Evaluating pCR after neoadjuvant systemic treatment of invasive breast cancer using DWI in comparison to DCE-based kinetic analysis
Rie Ota1, Masako Kataoka1, Maya Honda1, Mami Iima1, Kanae Kwai Miyake1, Akane Ohashi2, Yosuke Yamada3, Masakazu Toi4, and Yuji Nakamoto1
1Department of Diagnostic Imaging and Nuclear Medicine, Kyoto University graduate school of medicine, Kyoto, Japan, 2Kyoto Medical Center, Kyoto, Japan, 3Department of Pathology, Kyoto University Hospital, Kyoto, Japan, 4Department of Breast Surgery, Kyoto University Hospital, Kyoto, Japan
This study aimed to examine the performance of DWI in diagnosing pCR before surgery. Kinetic analysis from standard DCE-MRI were analyzed for comparison. ROC analysis for diagnosing pCR based on DWI score/Kinetic score by two readers was performed. Kinetic score showed slightly higher AUC while 95% confidence interval overlapped with that of DWI score. Both kinetic score and DWI score demonstrated excellent diagnostic performance among triple negative subtype compared to other subtypes.
|
|
0057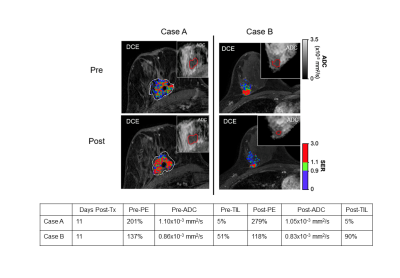 |
Multiparametric MRI signatures of immune response in patients with HER2+ breast cancer treated with trastuzumab Video Permission Withheld
Bonny Chau1, Debosmita Biswas1, Anum S. Kazerouni1, Daniel S. Hippe1, Rebeca Alvarez1, Suzanne Dintzis1, Laura C. Kennedy2, Vijayakrishna Gadi3, and Savannah C. Partridge 1
1University of Washington, Seattle, WA, United States, 2Vanderbilt University, Nashville, TN, United States, 3University of Illinois, Chicago, IL, United States
We investigated the relationship between immune infiltration and imaging metrics derived from breast MRI in patients with HER2+ breast cancer treated with trastuzumab. Fourteen patients with localized HER2+ breast cancer were imaged with diffusion-weighted and dynamic contrast-enhanced (DCE-) MRI prior to and ~2 weeks after a run-in dose of trastuzumab. Pre-treatment ADC and change in DCE-MRI peak percent enhancement were both significantly associated with immune response as characterized by change in level of tumor infiltrating lymphocytes.
|
||
0058.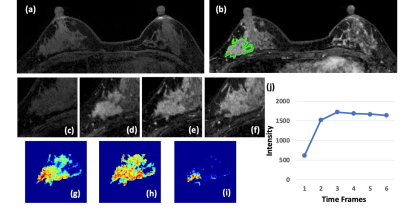 |
BI-RADS Reading of Non-Mass Lesions on DCE-MRI and Differential Diagnosis Performed by Radiomics and Deep Learning
Jiejie Zhou1, Yan-Lin Liu2, Yang Zhang2, Jeon-Hor Chen2,3, Freddie J. Combs2, Ritesh Parajuli4, Rita S. Mehta4, Huiru Liu1, Zhongwei Chen1, Youfan Zhao1, Meihao Wang1, and Min-Ying Su2
1Department of Radiology, First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China, 2Department of Radiological Sciences, University of California, Irvine, CA, United States, 3Department of Radiology, E-Da Hospital and I-Shou University, Kaohsiung, Taiwan, 4Department of Medicine, University of California, Irvine, CA, United States
A total of 150 lesions, 104 malignant and 46 benign, presenting as non-mass-like enhancements were analyzed. Three radiologists performed BI-RADS reading for the morphological distribution and internal enhancement pattern. For each case, the 3D tumor mask was generated using Fuzzy-C-Means segmentation. Three DCE parametric maps were generated, and PyRadiomics was applied to extract features. The radiomics model was built using 5 different machine learning algorithms. ResNet50 was implemented using three parametric maps as input. SVM yielded the highest accuracy of 80.4% in training, 77.5% in testing datasets. ResNet50 had better diagnostic performance, 91.5% in training, and 83.3% in testing datasets.
|
||
 |
0059.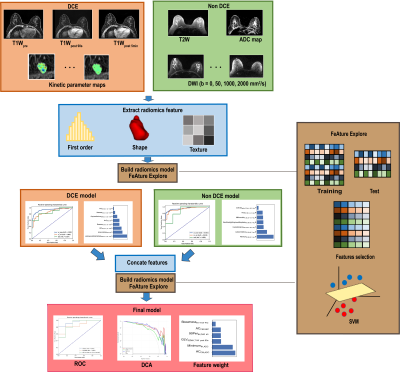 |
Radiomics based classification of breast mass with a multiparametric MRI protocol with DCE-MRI, T2, and DWI
Jing Zhang1, Chenao Zhan2, Tao Ai2, Xu Yan3, and Guang Yang1
1Shanghai key lab of magnetic resonance, shanghai, China, 2Tongji Medical College, Huazhong University of Science and Technology, Department of Radiology,Tongji Hospital, Wuhan, Hubei Province, China, 3Siemens Healthcare, MR Scientific Marketing, shanghai, China
DCE is the most useful MRI sequence for breast cancer diagnosis, but it often suffers from a high rate of false positive. To overcome this problem, we combined radiomics features from DCE, T2W, and DWI images to build a new machine learning model for differentiation of breast cancer. Our model achieved an AUC of 0.948 in an internal test cohort and 0.944 in an external test cohort, and reduced the false positive rate effectively. It was also found, first-order and texture features from ADC map made significant contributions to the model, suggesting the value ADC in breast cancer classification.
|
|
0060.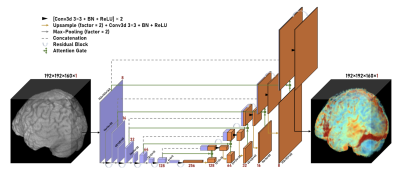 |
Predicting Gadolinium Contrast Enhancement for Structural Lesion Analysis using DeepContrast
Dipika Sikka1,2, Nanyan Zhu3, Chen Liu4, Scott Small5, and Jia Guo6
1Department of Biomedical Engineering, Columbia University, New York, NY, United States, 2VantAI, New York, NY, United States, 3Department of Biological Sciences and the Taub Institute, Columbia University, New York, NY, United States, 4Department of Electrical Engineering and the Taub Institute, Columbia University, New York, NY, United States, 5Department of Neurology, the Taub Institute, the Sergievsky Center, Radiology and Psychiatry, Columbia University, New York, NY, United States, 6Department of Psychiatry, Mortimer B. Zuckerman Mind Brain Behavior Institute, Columbia University, New York, NY, United States
Gadolinium-based contrast agents (GBCAs) have facilitated an improved analysis and understanding of structural lesions, however, present safety risks due to the tissue retention of GBCAs. Here we optimize and apply the deep learning model, DeepContrast, to predict gadolinium uptake in brain and breast structural lesions for structural lesion enhancement. The optimized DeepContrast models predict gadolinium uptake that is comparable to ground-truth scans consisting of the uptake from the GBCAs, using a single T1-weighted pre-contrast scan.
|
||
0061.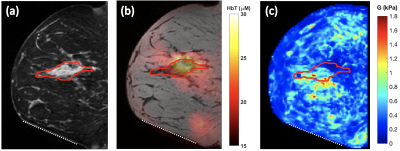 |
Multimodal magnetic resonance elastography and optical imaging of breast cancer
Bin Deng1,2,3, Mansi Saksena2,3, Steven Jay Isakoff3,4, Ralph Sinkus5, Samuel Patz3,6, and Stefan Alexandru Carp1,2,3
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Cancer Center, Massachusetts General Hospital, Boston, MA, United States, 5Laboratory for Vascular Translational Science (LVTS), Institut National de la Santé et de la Recherche Médicale (INSERM), Paris, France, 6Department of Radiology, Brigham and Women’s Hospital, Boston, MA, United States
Breast cancers are complex, evolving systems characterized by profound spatial and temporal heterogeneity in their biological nature. A multimodal multiparametric approach is needed to synergistically use imaging methods with different biophysical basis to simultaneously quantify multiple aspects of tumor physiology. We built a custom breast coil to allow multimodal near-infrared diffuse optical tomography and MR elastography imaging of human breast. Results of a three-inclusion dual-contrast phantom showed clear contrasts in reconstructed mechanical and optical properties as expected. Data on a breast cancer patient showed collocated hemoglobin and stiffness contrast at the tumor location.
|
||
0062.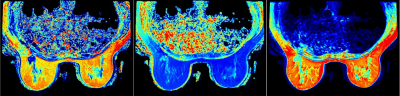 |
Radiomics model based on MAGIC acquisition for predicting neoadjuvant systemic treatment response in triple-negative breast cancer.
Nabil Elshafeey1, Gaiane M. Rauch2, Aikaterini Kotrotsou3, Beatriz E. Adrada1, Rosalind P. Candelaria1, Abeer H. Abdelhafez1, Huiqin Chen4, Jia Sun4, Medine Boge1, Rania M. M Mohamed1, Benjamin C. Musall5, Jong Bum Son5, Shu Zhang6,
Jason B. White7, Brandy Willis5, Elizabeth Ravenberg7, Wei Peng4, Stacy L. Moulder7, Wei Yang1, Mark D. Pagel6, Jingfei Ma5, and Ken-Pin Hwang5
1Breast Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Breast and Abdominal imaging, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 3The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 4Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 5Imaging Physics, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 6Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 7Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Early identification of treatment response to neoadjuvant systemic therapy (NAST) in Triple Negative Breast Cancer (TNBC) patients is important for appropriate treatment selection and response monitoring. In this study we evaluated the ability of a radiomic model extracted from a novel sequence, Magnetic Resonance Image Compilation (MAGIC), acquired before treatment initiation, to predict NAST response in TNBC. Our results showed that the radiomic signature derived from MAGIC maps (T1, PD and T2) can help differentiate responders from non-responders at baseline evaluation.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.