Oral
Improving Susceptibility Mapping: Greater Speed, Information & Accuracy
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 2 | 16:00 - 18:00 | Moderators: Chunlei Liu & Hongfu Sun |
 |
0787.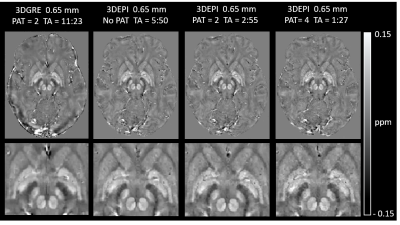 |
Submillimeter, Sub-Minute Quantitative Susceptibility Mapping using a Multi-Shot 3D-EPI with 2D CAIPIRINHA Acceleration
Monique Tourell1,2, Jin Jin2,3, Ashley Stewart1,2, Saskia Bollmann1, Steffen Bollmann1,2,4, Simon Robinson1,5,6, Kieran O'Brien2,3, and Markus Barth1,2,4
1Centre for Advanced Imaging, University of Queensland, Brisbane, Australia, 2ARC Training Centre for Innovation in Biomedical Imaging Technology, University of Queensland, Brisbane, Australia, 3Siemens Healthcare Pty Ltd, Brisbane, Australia, 4School of Information Technology and Electrical Engineering, University of Queensland, Brisbane, Australia, 5High Field Magnetic Resonance Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 6Department of Neurology, Medical University of Graz, Graz, Austria
High-resolution Quantitative Susceptibility Mapping (QSM) has the potential to improve multiple sclerosis imaging and pre-surgical planning for deep brain stimulation. Using a conventional 3D-gradient echo sequence, imaging times for submillimeter scans can be as long as 8 to 15 minutes. In this work, we implemented a multi-shot 3D-EPI sequence combined with 2D CAIPIRINHA acceleration to achieve high-quality susceptibility maps, with no visible distortions, at 0.80 mm and 0.65 mm isotropic resolutions in 58 and 87 seconds, respectively. Multi-echo 3D-GRE sequences producing similar QSMs required up to a 9-fold increase in acquisition time.
|
|
 |
0788.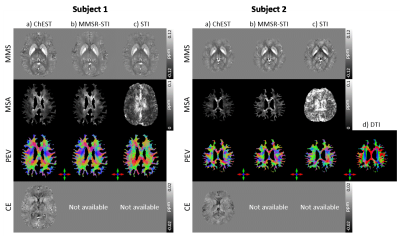 |
ChEST: A novel model measuring both Chemical Exchange and Susceptibility Tensor from resonance frequency shift
Hwihun Jeong1, Hyeong-Geol Shin1, Xu Li2, Sooyeon Ji1, and Jongho Lee1
1Department of Electrical and Computer Engineering, Seoul National University, Seoul, Korea, Republic of, 2Department of Radiology and Radiological Science, Division of MR Research, Johns Hopkins Medicine, Baltimore, MD, United States
We introduce ChEST, a novel model that can estimate both chemical exchange and magnetic susceptibility tensor effects from resonance frequency shift. For reconstruction, an iterative algorithm is designed to solve the inverse problem of the new model. When tested using numerical simulation datasets, our method successfully generated mean magnetic susceptibility, magnetic susceptibility anisotropy, principal eigenvector, and chemical exchange maps in high accuracy as compared to conventional methods. Application to in-vivo human brain was conducted, revealing promising outcomes.
|
|
0789.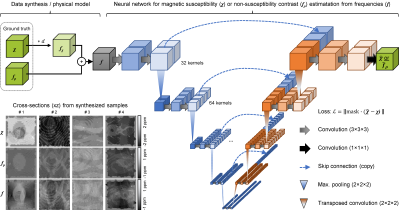 |
Quantitative mapping of susceptibility and non-susceptibility frequency with DEEPOLE QUASAR
Thomas Jochmann1, Dejan Jakimovski2, Nora Küchler1, Robert Zivadinov2,3, Jens Haueisen1, and Ferdinand Schweser2,3
1Department of Computer Science and Automation, Technische Universität Ilmenau, Ilmenau, Germany, 2Buffalo Neuroimaging Analysis Center, Department of Neurology at the Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States, 3Center for Biomedical Imaging, Clinical and Translational Science Institute, University at Buffalo, The State University of New York, Buffalo, NY, United States
Besides magnetic susceptibility, MRI phase contrast is caused by chemical exchange, anisotropic magnetic susceptibility, and anisotropic microstructural compartmentalization. These additional contributions are neglected by conventional QSM. This work presents an improved version of DEEPOLE QUASAR, a phase processing method that accounts for non-susceptibility signal contributions. We present preliminary results from studies on mice, volunteers, and multiple sclerosis (MS) patients.
|
||
 |
0790.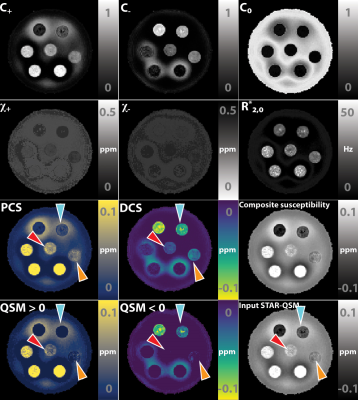 |
Decompose QSM to diamagnetic and paramagnetic components via a complex signal mixture model of gradient-echo MRI data
Jingjia Chen1, Nan-Jie Gong2, and Chunlei Liu1,3
1Department of Electrical Engineering and Computer Sciences, University of California, Berkeley, Berkeley, CA, United States, 2Vector Lab for Intelligent Medical Imaging and Neural Engineering, International Innovation Center of Tsinghua University, Shanghai, China, 3Helen Wills Neuroscience Institute, University of California, Berkeley, Berkeley, CA, United States
We propose and develop a method to separate paramagnetic and diamagnetic components within one voxel based on a 3-pool signal model. Alternating between solving for linear and nonlinear parameters in the model, paramagnetic component susceptibility (PCS) and diamagnetic component susceptibility (DCS) maps are constructed to represent the sub-voxel compartments.
|
|
 |
0791.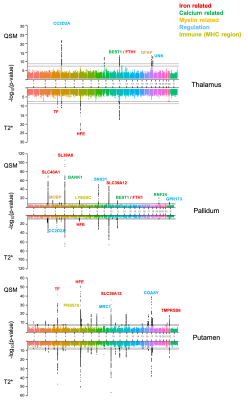 |
Genetic associations of magnetic susceptibility in the brain
Chaoyue Wang1, Benjamin C. Tendler1, Stephen M. Smith1, Fidel Alfaro-Almagro1, Alberto Llera2, Cristiana Fiscone3,4, Richard Bowtell3, Lloyd T. Elliott5, Karla L. Miller1, and Aurea B. Martins-Bach1
1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 2Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Nijmegen, Netherlands, 3Sir Peter Mansfield Imaging Centre, School of Physics and Astronomy, University of Nottingham, Nottingham, United Kingdom, 4Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy, 5Department of Statistics and Actuarial Science, Simon Fraser University, Vancouver, BC, Canada
UK Biobank is scanning 100,000 participants using multi-modal MRI (including swMRI). This rich resource also includes genome-wide characterisation of individuals. This provides a powerful opportunity to relate swMRI-derived measures to genetics. The aim of this work is to identify genetic associations of QSM-based measures. We carried out genome-wide association studies (GWAS) in over 30,000 subjects for both QSM and T2* based measures. Our results demonstrate that susceptibility and T2* measures are associated with genetic loci involved in a range of biological functions. Susceptibility and T2* IDPs share many common genetic associations but there are also complementary associations.
|
|
 |
0792.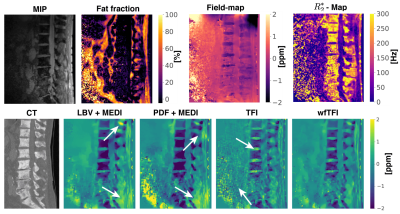 |
Preconditioned water–fat total field inversion: application to spine quantitative susceptibility mapping (QSM)
Christof Boehm1, Nico Sollmann2,3,4, Jakob Meineke5, Sophia Kronthaler1, Stefan Ruschke1, Michael Dieckmeyer2,3, Kilian Weiss6, Claus Zimmer2,3, Marcus R. Makowski1, Thomas Baum2,3, and Dimitrios C. Karampinos1
1Department of Diagnostic and Interventional Radiology, School of Medicine, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 2Department of Diagnostic and Interventional Neuroradiology, School of Medicine, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 3TUM-Neuroimaging Center, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 4Department of Diagnostic and Interventional Radiology, University Hospital Ulm, Ulm, Germany, 5Philips Research Lab, Hamburg, Germany, 6Philips Healthcare, Hamburg, Germany
Body-QSM remains challenging for various reasons including, (a) the incomplete separation of background- and local-fields included by assumed approximations,(b) incorrect modeling at low-SNR-voxels included by linear modeling of the inverse problem and (c) streaking artifacts. A recently developed total-field-inversion (TFI) QSM method that directly estimates the susceptibility from multi-echo data showed significant alleviation of the above artifacts. However, the original TFI method can only be applied to regions with one species. This work proposes a QSM method that directly estimates susceptibility from multi-echo data in regions where water and fat are present and demonstrates its advantages over former proposed methods.
|
|
 |
0793.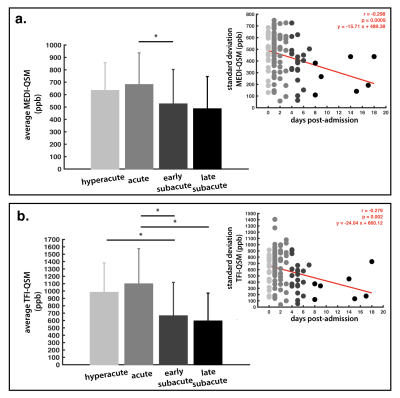 |
Quantitative susceptibility imaging to stage acute cerebral hemorrhages: A direct comparison of the mcTFI and MEDI methods
Allen A Champagne1,2, Yan Wen3, Magdy Selim4, Aristotelis Filippidis 5, Ajith Thomas5, Pascal Spincemaille3, Yi Wang3, and Salil Soman 6
1School of Medicine, Queen's University, Kingston, ON, Canada, 2Center for Neuroscience Studies, Queen's University, Kingston, ON, Canada, 3Radiology, Weill Cornell Medicine, New York, NY, United States, 4Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 5Neurosurgery, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States, 6Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
The perceived acuity (hyperacute, acute, subacute) of intracerebral hemorrhage (ICH) dramatically impacts patient management. While CT and standard MRI are limited for staging ICH, Quantitative Susceptibility Imaging (QSM) has shown promising results for tracking the evolution of ICH, as a substrate for the pathophysiology within bleeds. Here, we compare novel multi-echo complex total field inversion (mcTFI) QSM for staging ICHs, in comparison to conventional Morphology Enabled Dipole Inversion (MEDI). mcTFI better distinguished hyperacute/acute timepoints from subacute ICHs, in comparison to MEDI, likely as a result of the robust inversion computation, inherently reducing susceptibility quantification errors and shadowing artifacts.
|
|
 |
0794.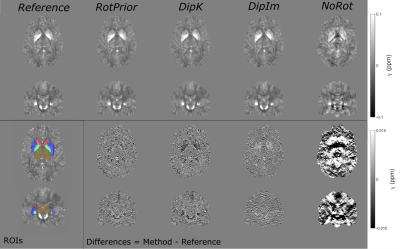 |
The Effect of Oblique Image Slices on the Accuracy of Quantitative Susceptibility Mapping and a Robust Tilt Correction Method
Oliver C. Kiersnowski1, Anita Karsa1, John S. Thornton2, and Karin Shmueli1
1Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 2UCL Queens Square Institute of Neurology, London, United Kingdom
Quantitative susceptibility mapping (QSM) using the MRI phase to calculate tissue magnetic susceptibility is finding increasing clinical applications. Oblique image slices are often acquired to facilitate radiological viewing and reduce artifacts. Here, we show that artifacts and errors arise in susceptibility maps if oblique acquisition is not properly taken into account in QSM. We performed a comprehensive analysis of the effects of oblique acquisition on brain susceptibility maps and compared tilt correction schemes for three susceptibility calculation methods, using a numerical phantom and human in-vivo images. We demonstrate a robust tilt correction method for accurate QSM with oblique acquisition.
|
|
0795.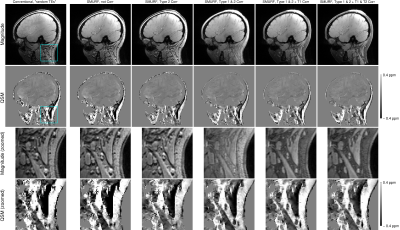 |
QSM of the head-and-neck at 7T using simultaneous fat-water imaging with SMURF
Beata Bachrata1,2, Korbinian Eckstein1, Siegfried Trattnig1,2, and Simon Daniel Robinson1,3,4
1High Field MR Centre, Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna, Austria, 2Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria, 3Centre of Advanced Imaging, University of Queensland, Brisbane, Australia, 4Department of Neurology, Medical University of Graz, Graz, Austria
We address the challenges of QSM in regions outside of the brain which contain disconnected structures - leading to difficulty in masking - and significant amounts of fat with chemical shift and relaxation differences relative to water - leading to errors in susceptibility estimates. We propose a mask generation approach based on signal phase and show that the errors in susceptibility estimates can be eliminated using simultaneous fat-water imaging with SMURF. Using multi-echo acquisition and inverse-variance-weighted echo combination, we generate high CNR, chemical shift and relaxation effects-free susceptibility maps of the head-and-neck at 7T.
|
||
0796.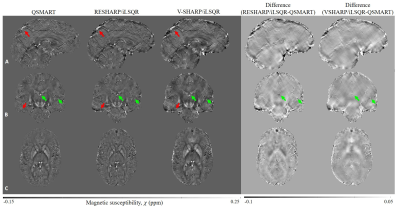 |
QSMART: a parallel stage QSM pipeline for suppression of cortical and venous artifacts
Negin Yaghmaie1,2, Warda Syeda3,4, Chengchuan Wu1,2, Yicheng Zhang1,2, Bradford A. Moffat1,4, Rebecca Glarin1,5, Scott Kolbe4,6,7, and Leigh Johnston1,2
1Melbourne Brain Centre Imaging Unit, The University of Melbourne, Melbourne, Australia, 2Department of Biomedical Engineering, The University of Melbourne, Melbourne, Australia, 3Melbourne Neuropsychiatry Centre, The University of Melbourne, Melbourne, Australia, 4Department of Medicine and Radiology, The University of Melbourne, Melbourne, Australia, 5Department of Radiology, Royal Melbourne Hospital, Melbourne, Australia, 6Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Australia, 7Department of Radiology, Alfred Hospital, Melbourne, Australia
We propose a two-stage QSM method, Quantitative Susceptibility Mapping Artifact Reduction Technique (QSMART), in which tissue and vein susceptibility values are estimated in parallel by generating a vasculature mask from the magnitude data using a Frangi filter. Spatially Dependent Filtering is employed for the background field removal and the two susceptibility estimates are combined in the final QSM map. QSMART is compared to RESHARP/iLSQR and V-SHARP/iLSQR inversion on 7T in vivo single and multiple-orientation scans. QSMART demonstrates superior artifact suppression in the cortex and near vasculature, and is a robust tool for susceptibility estimation. QSMART code is available at https://github.com/MBCIU/QSMART.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.