Oral
CEST, MT & T1ρ
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 4 | 16:00 - 18:00 | Moderators: |
 |
0145.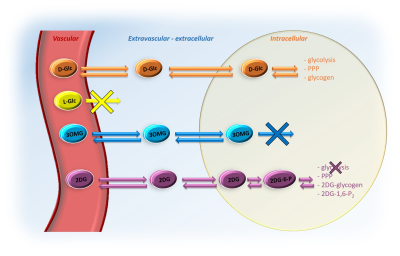 |
Elucidating the compartmental origin of glucoCEST signal using glucose analogues
Yohann Mathieu-Daudé1, Mélissa Vincent1, Julien Valette1, and Julien Flament1
1Université Paris-Saclay, Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), Centre National de la Recherche Scientifique (CNRS), Molecular Imaging Research Center (MIRCen), Laboratoire des Maladies Neurodégénératives, Fontenay-aux-Roses, France
The compartmental origin of glucoCEST signal is still an ongoing debate. To address this crucial question, we proposed in this study to intravenously inject natural D-glucose and several metabolizable and non-metabolizable glucose analogues to compare their relative glucoCEST signal kinetics. The accurate measurements of glucoCEST signal kinetics provided deeper insights into the origin of glucoCEST signal and constitute a major step toward quantitative measurement of glucose metabolism using CEST imaging method. This could provide a new non-invasive tool to study brain energy metabolism defects observed in numerous neurodegenerative disorders.
|
|
0146.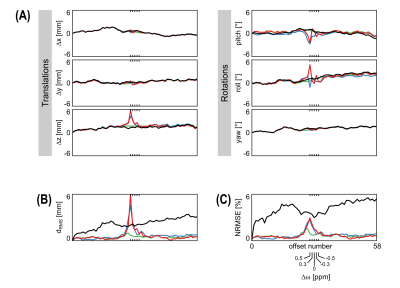 |
Motion correction for 3D CEST imaging without direct water saturation artefacts
Johannes Breitling1, Andreas Korzowski1, Neele Kempa1, Philip S. Boyd1, Mark E. Ladd1, Peter Bachert1, and Steffen Goerke1
1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
In CEST-MRI, motion correction is compromised by the drastically changing image contrast at different frequency offsets and particularly at the direct water saturation. In this study, a simple extension for conventional image registration algorithms is proposed, enabling a robust and accurate motion correction of CEST-MRI data. Performance of different approaches was investigated using a ground truth dataset, generated from repeated 3D in vivo measurements at 3 T, corrupted with realistic random rigid motion patterns and noise. In comparison to the conventional image registration and a cutting-edge algorithm specifically developed for CEST-MRI, the proposed method achieved more accurate and robust results.
|
||
0147.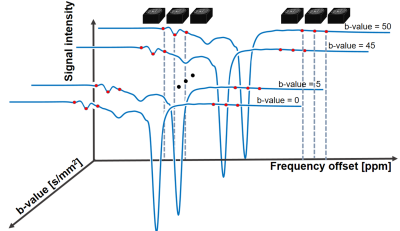 |
Diffusion-weighted Chemical Exchange Saturation Transfer Imaging at 7T Human MRI
Yujin Jung1, Jaeseok Park2, Seong-Gi Kim2, and Sung-Hong Park1
1Department of Bio and Brain engineering, Korea Advanced Institute of Science and Technology, Daejeon, Korea, Republic of, 2Department of Global Biomedical Engineering, Sungkyunkwan University, Suwon, Korea, Republic of
CEST is a useful method to diagnose tumor or stroke, but lacks physical information such as compartments with different diffusivities. In this study, we developed a new diffusion-weighted steady-state CEST sequence using 3D EPI at 7T. The technique was tested in phantom and human brain, and the preliminary CEST-weighted apparent diffusion coefficient maps provided both CEST and diffusion information. Further study is required to clearly understand the signal source and the potential as a new biomarker.
|
||
0148.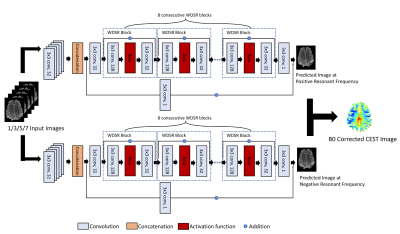 |
Deep Learning Enables A Half Z-spectrum Sampling-based B0 Inhomogeneity Correction for CEST MRI
Yiran Li1, Danfeng Xie1, Dushyant Kumar2, Abigail Cember2, Ravi Prakash Reddy Nanga2, Hari Hariharan2, John A. Detre3, Ravinder Reddy2, and Ze Wang1
1Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Department of Radiology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States, 3Department of Neurology, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States
This study presents a DL based framework for correcting B0 inhomogeneity for GluCEST imaging using fewer acquisitions. Based on 3 or 5 positive offset CEST images, the proposed method can save >80% of CEST imaging acquisition time as compared to current 26 pairs of double site z-spectrum irradiations based protocol. This approach can be applied to other CEST imaging as well.
|
||
0149.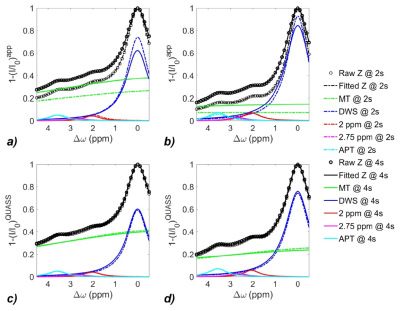 |
Quasi-steady-state (QUASS) CEST for robust quantification of tumor MT and APT effects by correction of saturation time and relaxation delay
Xiao-Yong Zhang1, Botao Zhao1, Zhe Phillip Sun2, and Yin Wu3
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Yerkes National Primate Research Center, Emory University, Atlanta, GA, United States, 3Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
To reduce CEST measurement’s dependence on long RF saturation duration (Ts) and relaxation delay (Td), we developed a post-processing strategy to derive the quasi-steady-state (QUASS) CEST from apparent measurements. The simulation and in-vivo experiment results show that the apparent MT and APT effects and their contrast substantially depend on Ts and Td. In comparison, the QUASS MT and APT effects and their difference between contralateral normal tissue and tumor exhibit little dependence on Ts and Td. To conclude, the QUASS CEST algorithm enables robust CEST quantification and offers a straightforward approach to standardize CEST measurements.
|
||
0150.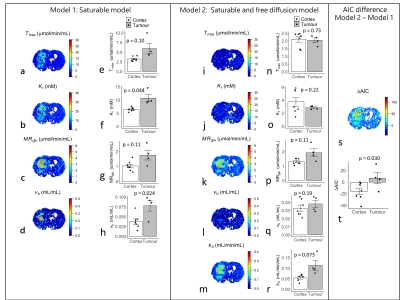 |
Non-invasive mapping of cerebral glucose transport and metabolism using glucoCESL MRI
Ben R Dickie1,2, Tao Jin3, Rainer Hinz4, Geoff JM Parker5,6, Laura M Parkes1,2, and Julian Matthews1,2
1Division of Neuroscience and Experimental Psychology, Faculty of Biology Medicine and Health, The University of Manchester, Manchester, United Kingdom, 2Geoffrey Jefferson Brain Research Centre, Manchester Academic Health Science Centre, Manchester, United Kingdom, 3Department of Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 4Division of Informatics, Imaging, and Data Sciences, Faculty of Biology, Medicine, and Health, The University of Manchester, Manchester, United Kingdom, 5Centre for Medical Image Computing, Department of Computer Science and Department of Neuroinflammation, University College London, London, United Kingdom, 6Bioxydyn Ltd, Manchester, United Kingdom
Chemical-exchange spin-lock (CESL) MRI can detect uptake and clearance of intravenously administered glucose into the brain at high spatial resolution. We apply quantitative modelling to describe glucoCESL kinetics in tumour-bearing and healthy rats. Parameters relating to glucose transport (Tmax, Kt, kd), metabolism (MRglu) and blood volume (vb) were estimated and compared between tumour and cortical tissue. Kinetic modelling of glucoCESL MRI data yields meaningful estimates of glucose transport and metabolism, and our modelling approach holds great promise to probe glucose transport and metabolism at high spatial resolution.
|
||
0151.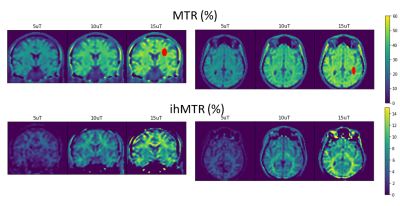 |
Inhomogeneous Magnetization Transfer Steady State Imaging at 0.5T: Exploring SAR and B1+RMS envelope.
Andrew T Curtis1 and Chad T Harris1
1Research and Development, Synaptive Medical, Toronto, ON, Canada
A balanced steady state sequence with multiband saturation pulses was implemented on a 0.5T scanner to assess the potential for additional inhomogeneous magnetization transfer contrast generation from the higher SAR and B1+RMS limits. Volumes were acquired with B1+RMS saturation less than 15uT. Initial results are promising with ihMT contrast scaling nearly linearly with applied B1+ as expected, achieving contrast levels of 12-16% in white matter for B1+RMS of 15uT. This linear contrast increase could directly offset losses in polarization efficiency from mid-field as compared to high field, providing an interesting application area with competitive CNR.
|
||
0152.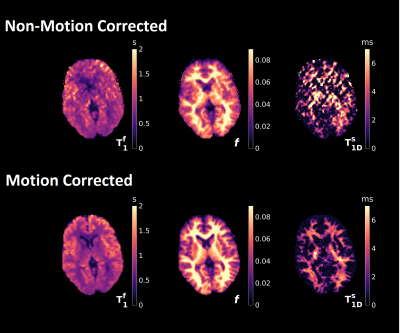 |
Motion corrected magnetization transfer-mediated fingerprinting (MT-MRF) using DISORDER.
Daniel J. West1, Lucilio Cordero-Grande1,2,3, Rui P. A. G. Teixeira1,2, Giulio Ferrazzi4, Joseph V. Hajnal1,2, and Shaihan J. Malik1,2
1Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Centre for the Developing Brain, King's College London, London, United Kingdom, 3Biomedical Image Technologies, ETSI Telecomunicación, Universidad Politécnica de Madrid & CIBER-BNN, Madrid, Spain, 4IRCCS San Camilo Hospital, Venice, Italy
ihMT is a promising approach for myelin imaging due to its specificity to substances with non-zero dipolar order. However, tissue model quantification requires high resolution acquisitions in excess of twenty minutes in length and so necessitates the use of motion correction methods to prevent artefacts. In this work we combine our recent MT-mediated MRF sequence with the DISORDER retrospective motion correction method. This new framework can acquire and reconstruct high resolution motion-compensated 3D time-resolved data from fingerprinting sequences. Semi‑quantitative MT and ihMT ratio maps as well as quantitative maps of tissue parameters can be obtained from the resulting images.
|
||
0153.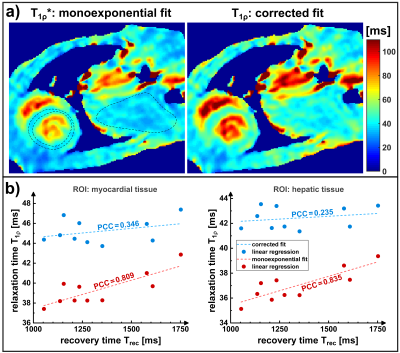 |
Formalism of the T1ρ* relaxation pathway: Correction of quantification errors for rapid myocardial T1ρ mapping in mice
Maximilian Gram1,2, Daniel Gensler1,3, Patrick Winter1,2, Fabian Gutjahr2,3, Michael Seethaler2,3, Peter Michael Jakob2, and Peter Nordbeck1,3
1Department of Internal Medicine I, University Hospital Würzburg, Würzburg, Germany, 2Experimental Physics 5, University of Würzburg, Würzburg, Germany, 3Comprehensive Heart Failure Center (CHFC), University Hospital Würzburg, Würzburg, Germany
The rapid quantification of T1ρ using fast gradient echo sequences for data acquisition leads to a contamination of the T1ρ relaxation pathway. Analogous to the T1* relaxation occurring in snapshot flash sequences, a relaxation pathway T1ρ* is effectively observed. As a consequence, quantification errors can arise depending on T1 and the sequence parameters used for imaging. In this work we introduce a formalism for the description of T1ρ* and present a method that can be applied for the subsequent correction of study results in the field of cardiac MRI.
|
||
0154.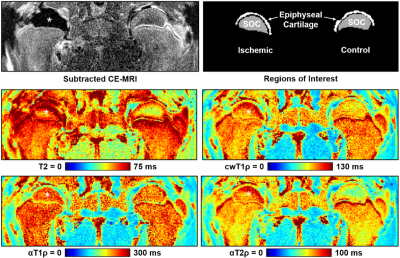 |
Utility of Adiabatic T1ρ and T2ρ Mapping to Detect Ischemic Injury to the Femoral Head: An In Vivo Piglet Model Study at 3T MRI
Casey P. Johnson1,2, Sampada Bhave1, Alexandra R. Armstrong1, and Ferenc Toth1
1Department of Veterinary Clinical Sciences, University of Minnesota, Saint Paul, MN, United States, 2Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
Adiabatic T1ρ and T2ρ are potentially advantageous relaxation time mapping techniques for in vivo imaging of the musculoskeletal system. In this work, we compared adiabatic T1ρ and T2ρ mapping to T2 and continuous-wave T1ρ mapping in a piglet model of osteonecrosis of the femoral head. We found that adiabatic T2ρ had a robust increase in response to ischemic injury to the bone marrow, bone, and epiphyseal cartilage of the ischemic femoral head compared to the contralateral healthy femoral head. Adiabatic T2ρ may be a useful technique to detect early-stage injury in ischemic bone and joint disorders.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.