Oral
Quantitative Neuroimaging
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 5 | 14:00 - 16:00 | Moderators: Chaitra Badve & Dharmesh Tailor |
 |
0093.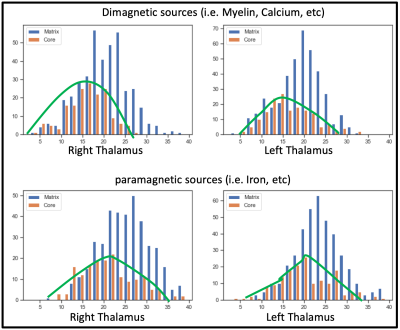 |
Mapping of Thalamic Matrix and Core Nuclei using QSM at 9.4 Tesla
Vinod Jangid Kumar1, Klaus Scheffler1,2, Gisela E Hagberg1,2, and Wolfgang Grodd1
1Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Biomedical Magnetic Resonance, University Hospital and Eberhard-Karl’s University, Tuebingen, Germany
The thalamus is a central connectivity hub of the human brain that remains poorly understood concerning its anatomy. Since it houses both calcium-rich neurons and myelin-rich architecture, quantitative susceptibility mapping at the ultra-high-field may facilitate thalamic substructures' characterization. Consequently, we have acquired high-resolution QSM data at 9.4 Tesla in 21 subjects and analyzed human thalamic nuclei with respect to core and matrix neurons. We found a more substantial contribution of both diamagnetic and paramagnetic sources, like iron, myelin, and calcium, in the matrix nuclei in contrast to the relay specific core nuclei matrix nuclei.
|
|
 |
0094.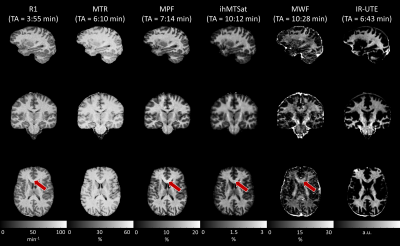 |
On Comparability and Reproducibility of Myelin Sensitive Imaging Techniques
Tom Hilbert1,2,3, Lucas Soustelle4, Gian Franco Piredda1,2,3, Thomas Troalen5, Stefan Sommer6,7, Arun Joseph8,9,10, Reto Meuli2, Jean-Philippe Thiran2,3, Guillaume Duhamel4, Olivier M. Girard4, and Tobias Kober1,2,3
1Advanced Clinical Imaging Technology (ACIT), Siemens Healthcare, Lausanne, Switzerland, 2Department of Radiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 3LTS5, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 4Aix Marseille Univ, CNRS, CRMBM, Marseille, France, 5Siemens Healthcare SAS, Saint-Denis, France, 6Siemens Healthcare, Zurich, Switzerland, 7Swiss Center for Musculoskeletal Imaging (SCMI), Balgrist Campus, Zurich, Switzerland, 8Advanced Clinical Imaging Technology (ACIT), Siemens Healthcare, Bern, Switzerland, 9Translational Imaging Center, Sitem-Insel, Bern, Switzerland, 10Departments of Radiology and Biomedical Research, University of Bern, Bern, Switzerland
A reliable and non-invasive measurement of myelin content in the brain is of high importance for neurodegenerative diseases such as multiple sclerosis. To this end, various methods have been developed over the past years with different advantages and shortcomings. In this work, six widely used methods are compared and tested for reproducibility: (i) longitudinal relaxation rate, (ii) magnetization transfer ratio, (iii) macromolecular proton fraction, (iv) inhomogeneous magnetization transfer saturation, (v) myelin water fraction, and (vi) inversion recovery at ultra-short echo time. This comparison may facilitate an informed decision on which myelin imaging techniques should be used in future studies.
|
|
 |
0095.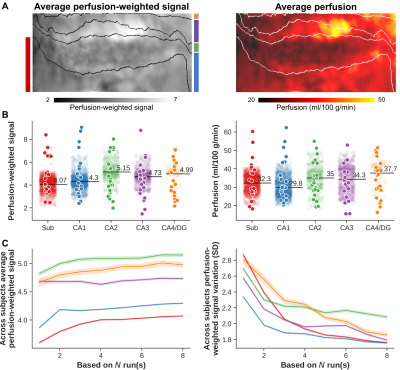 |
Delineating perfusion and the effects of vascularisation patterns across the hippocampal subfields at 7T
Roy AM Haast1, Sriranga Kashyap2, Mohamed D Yousif1, Dimo Ivanov2, Benedikt A Poser2, and Ali R Khan1
1Centre for Functional and Metabolic Mapping, Western University, London, ON, Canada, 2Maastricht University, Maastricht, Netherlands
Intra-hippocampal perfusion patterns have remained unexplored so far but might carry important information to study disease pathogenesis. In this study we used arterial spin labeling (ASL) combined with time-of-flight angiography acquired at 7T to delineate perfusion and the effects of vascularisation patterns across the hippocampal subfields. We found that (i) imaging hippocampal perfusion in vivo is feasible using ASL at 7T, (ii) perfusion could serve as biomarker to differentiate hippocampal subfields, but that (iii) the impact of the dense macrovascular network varies based on characteristics such as distance and diameter.
|
|
 |
0096.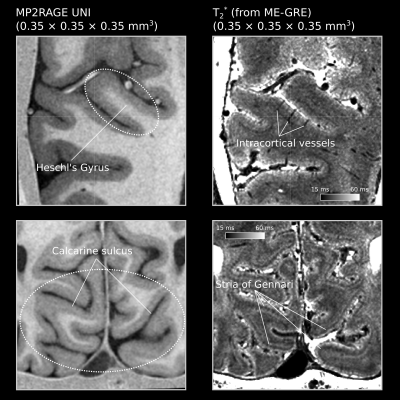 |
In vivo human T2* imaging at 0.35 mm reveals up to 15 ms of local variations within gray matter across depths at 7T
Omer Faruk Gulban1,2, Saskia Bollman3, Renzo Huber1, Kendrick Kay4, Benedikt Poser1, Federico De Martino1, and Dimo Ivanov1
1Department of Cognitive Neuroscience , Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands, 2Brain Innovation, Maastricht, Netherlands, 3Centre for Advanced Imaging, The University of Queensland, Brisbane, Australia, 4Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States
We measured in vivo human brain T2* values using 7T MRI at 0.35 × 0.35 × 0.35 mm3. We simultaneously targeted calcarine sulcus and Heschl’s gyrus. Our results show that gray matter T2* varies up to 15 ms (global range being mostly in between 25-45 ms) from deep to superficial layers. The stria of Gennari shows up as a major reduction of T2* within calcarine sulcus. However, a similar layering is not visible within Heschl’s gyrus. B0 alignment effects seem to be not as strong as the biological tissue composition effects that are observed across the visual and auditory regions.
|
|
0097.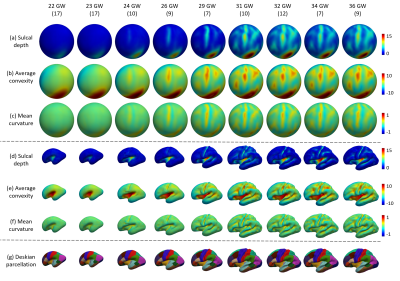 |
Construction of Spatiotemporal Cortical Surface Atlases for Fetal Brains
Zhengwang Wu1, Yuchen Pei1, Ya Wang1, Tao Zhong1, Fenqiang Zhao1, Li Wang1, He Zhang2, and Gang Li1
1Department of Radiology and BRIC, UNC-Chapel Hill, Chapel Hill, NC, United States, 2Department of Radiology, Obstetrics and Gynecology Hospital, Fu Dan University, Shanghai, China
We constructed a set of temporally-densely sampled cortical surface atlases for the fetal brain from 22 to 36 gestational weeks. This 4D fetal cortical surface atlas, which will be released to the public soon, together with the UNC 4D Infant Cortical Surface Atlas provide the longest temporally-consistent atlas chain from the prenatal 22 gestational weeks to the postnatal 7 years of age.
|
||
0098.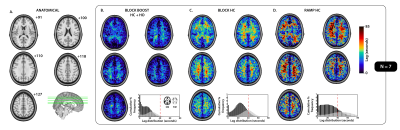 |
Combined blood flow and CO2-mediated effects underlie the tissue-specific response to hypercapnia: Insight from 7T MR-based imaging
Allen A Champagne1,2 and Alex A Bhogal3
1School of Medicine, Queen's University, Kingston, ON, Canada, 2Center for Neuroscience Studies, Queen's University, Kingston, ON, Canada, 3Radiology, University Medical Center Utrecht, Utrecht, Netherlands
Cerebrovascular reactivity (CVR) mapping is finding increasing clinical applications as a non-invasive probe for vascular health. Untangling physiological factors driving differences in temporal delays within the tissue-specific CVR response can help better understand the pathophysiological mechanisms associated with vascular impairments. Here, we combine hypercapnic and hyperoxic respiratory challenges with high resolution 7T MR-based imaging to gather insight about differences in the temporal response to CVR between grey- and white-matter tissues. Our findings support the hypothesis that differences in the physiological response to hypercapnia may be determined by compounding effects related to CO2 sensitivity and blood flow (re)distribution.
|
||
0099.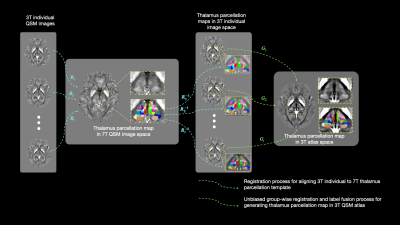 |
7T QSM guided Histologically Consistent Thalamic Sub-nucleus Parcellation in 3T QSM Atlas Space
Weimin Zhang1, Chenyu He1, Xiaojun Guan2, Xiaojun Xu2, Hongjiang Wei3, and Yuyao Zhang1,4,5
1School of Information Science and Technology, ShanghaiTech University, Shanghai, China, 2Department of Radiology, The Second Affiliated Hospital, Zhejiang, China, 3Institute for Medical Imaging Technology, School of Biomedical Engineering, Shanghai, China, 4Shanghai Engineering Research Center of Intelligent Vision and Imaging, ShanghaiTech University, Shanghai, China, 5iHuman Institute, ShanghaiTech University, Shanghai, China The thalamus is a relay station that routed and modulated brain signals from the deep gray-matter to the cortex. It can be divided into sub-nuclei that are highly related to various neurological disorders. However, those sub-nuclei are indistinguishable in standard T1 or T2 weighted MRI. We present a multi-atlas thalamic sub-nucleus parcellation framework guided by 7T QSM image, which provides histologically consistent contrast in thalamic sub-nuclei. Combining with a set of 3T QSM images, the thalamic parcellation in 7T image space is transferred into the 3T atlas space as a 64 sub-nucleus parcellation map. |
||
0100.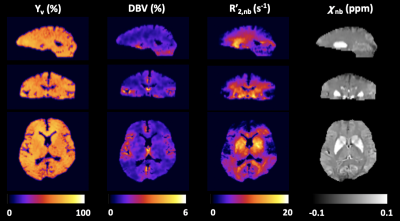 |
Whole-Brain 3D Quantitative BOLD Mapping With Preliminary Estimation of R2' and Venous Blood Volume
Hyunyeol Lee1 and Felix W Wehrli1
1Radiology, University of Pennsylvania, Philadelphia, PA, United States
Quantitative BOLD (qBOLD) seeks to quantify voxel-wise deoxygenated blood volume (DBV) and venous blood oxygen saturation (Yv) based on R2′-sensitive signal acquisitions. A complication in qBOLD is the separation of signal contributions from R2, and R2′ from heme and non-heme iron sources, particularly in the deep brain structures. Here, we develop a new 3D qBOLD mapping method by tackling the confounding factors based on preliminary estimates of R2, R2’, and voxel susceptibility, along with cerebral venous blood volume. Results suggest feasibility of the proposed, prior-based qBOLD method for 3D mapping of DBV and Yv across the entire brain.
|
||
 |
0101.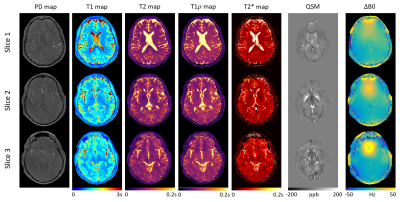 |
10min Whole-Brain MRI Solution – Comprehensive Quantification of MR Relaxometry and Susceptibility Plus Synthetic Contrast-Weighted Images
Sen Ma1, Tianle Cao1,2, Nan Wang1, Anthony G. Christodoulou1, Zhaoyang Fan1, Yibin Xie1, and Debiao Li1
1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States
We propose an integrated and efficient solution to clinical whole-brain MRI in a single 10min sequence, producing co-registered, quantitative PD, T1, T2, T1ρ, T2*, QSM, and ΔB0 information plus clinically adopted, synthetic contrast-weighted images including PDw, T1w, T2w, T2*w, FLAIR, SWI, true-SWI, mIP, and true-SWI mIP simultaneously. Quantitative maps and contrast-weighted images are generated with good image quality and contrasts. Quantitative measurements agree with literature values. This method has the clinical potential for comprehensive risk assessment and disease evaluation, combining early detection, diagnosis, tissue characterization, and treatment monitoring of various brain diseases.
|
|
0102.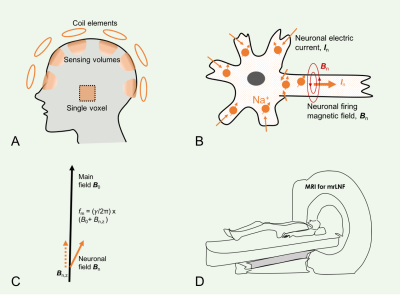 |
Magnetic resonance recording of local neuronal firings (mrLNF) in the human brain: A proof of concept
Yongxian Qian1, Karthik Lakshmanan1, Anli Liu2, Yvonne W. Lui1, and Fernando E. Boada1
1Radiology, New York University, New York, NY, United States, 2Neurology, New York University, New York, NY, United States
Neurons are firing when emitting action potentials to communicate with each other. Action potentials generate fast electric currents (~2ms duration) across membrane and slow ones (~10–100ms) at postsynaptic side. These currents generate electric and magnetic fields detectable by scalp EEG and MEG, respectively. They detect the fields relatively far away (~20mm) from firing sources and are only sensitive to slow, easily-synchronized postsynaptic currents. Here we propose a new approach termed as magnetic resonance recording of local neuronal firings (mrLNF) that has a very high temporal resolution (0.25ms) and can non-invasively detect fast and slow neuronal currents at the firing sources.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.