Oral
Signal Modelling for Quantitative MRI
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 1 | 14:00 - 16:00 | Moderators: Ileana Jelescu & Herbert Köstler |
 |
0493. |
Probing restricted diffusion and water exchange with free gradient waveforms: Addressing the need for a compartment model
Arthur Chakwizira1, Filip Szczepankiewicz2, Linda Knutsson1,3, and Markus Nilsson2
1Department of Medical Radiation Physics, Lund University, Lund, Sweden, 2Department of Diagnostic Radiology, Lund University, Lund, Sweden, 3Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Diffusion MRI can be used to probe restricted diffusion (compartment size) and water exchange (membrane permeability), but their effects on the signal are entangled. This problem can be addressed by the use of free gradient waveforms designed to disentangle the effects. However, a previously presented approach is invalid at high b-values and long diffusion encoding times. We develop a generalised restriction-exchange model that is valid for arbitrary gradient waveform and all relevant b-values and encoding times. The approach eliminates the shortcomings of the previous restriction-exchange framework.
|
|
 |
0494.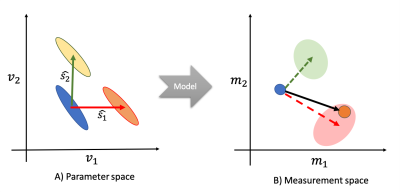 |
Identifying microstructural changes in diffusion MRI models; How to break parameter degeneracies
Hossein Rafipoor1, Saad Jbabdi1, Ludovica Griffanti1,2, and Michiel Cottaar1
1Wellcome Centre for Integrative Neuroimaging (WIN), Nuffield Department of Clinical Neurosciences (NDCN), University of Oxford, Oxford, United Kingdom, 2Wellcome Centre for Integrative Neuroimaging (WIN), Department of Psychiatry, University of Oxford, Oxford, United Kingdom
We present a novel Bayesian framework to relate changes in data to changes in model parameters even in models that cannot be directly inverted. We do so by training probabilistic models that characterise how the measurements change as a result of a change in the parameters. While the approach is general, in this work we used the framework to study microstructural parameter changes that are associated with the appearance of areas of white matter hyperintensities. We found a dichotomy between periventricular and deep white matter hyperintensities, where the latter are associated with increased extracellular signal.
|
|
 |
0495. |
Sensitivity of cortical kurtosis measurement to diffusion time in KINSA modeling assessed with Connectome scanner diffusion MRI
Tianjia Zhu1,2, Qiyuan Tian3,4, Susie Huang3,4, and Hao Huang1,5
1Department of Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 2Department of Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Radiology, Harvard Medical School, Boston, MA, United States, 4Massachusetts General Hospital, Boston, MA, United States, 5Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States
Unlike diffusion in the white matter which can be well characterized by Gaussian diffusion, substantial non-Gaussian diffusion occur in the cerebral cortical mantle featured by widespread barriers of both somas and neurites. We have developed a non-Gaussian compartmental model Kurtosis-based Imaging of Neurite and Soma Architecture (KINSA) in previous studies. Here, we demonstrate the sensitivity of cortical kurtosis to diffusion time in KINSA modeling with both simulated data and in-vivo dMRI data from Connectome scanner. The mean kurtosis sensitivity to diffusion time allows for precisely delineating neurite and soma architecture such as neuronal density and soma radius.
|
|
 |
0496.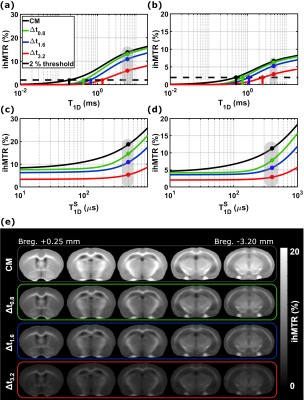 |
Inhomogeneous Magnetization Transfer (ihMT): theoretical characterization of T1D-filtering and experimental validation
Andreea Hertanu1,2, Lucas Soustelle1,2, Arnaud Le Troter1,2, Julie Buron1,2,3, Julie Le Priellec3, Victor N. D. Carvalho1,2,4, Myriam Cayre3, Pascale Durbec3, Gopal Varma5, David C. Alsop5, Olivier M. Girard1,2, and Guillaume Duhamel1,2
1Aix Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3Aix Marseille Univ, CNRS, IBDM, Marseille, France, 4Aix Marseille Univ, CNRS, ICR, Marseille, France, 5Division of MR Research, Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
Inhomogeneous magnetization transfer (ihMT) signal originates from the residual dipolar interactions and is weighted by the associated dipolar relaxation time T1D. The resulting signal can be modulated by filtering the contribution of short T1D components to emphasize the contrast between different structures, or to enhance the specificity for myelin imaging. In this study, the dependency of ihMTR to T1D is investigated theoretically for different T1D-filtering strengths. Experimental WM/GM relative contrasts for the same configurations are put in perspective with theoretical contrasts resulted from single-T1D and bi-T1D biophysical model simulations.
|
|
0497.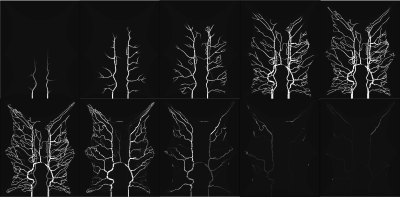 |
A computational fluid dynamics framework to generate digital reference objects for perfusion imaging
Ulin Nuha Abdul Qohar1, Erik Andreas Hanson1, Steven Sourbron2, and Antonella Zanna Munthe-Kaas1
1Mathematics, University of Bergen, Bergen, Norway, 2University of Sheffield, Sheffield, United Kingdom
In this study, we present a simulation framework capable of generating synthetic reference perfusion MRI data suitable for evaluation and comparison of tracer kinetic models. The framework consists of a graph-based contrast agent flow model with a vascular geometry and allows for controlled simulations with realistic structural and vascular parameters. We demonstrate the potential application of the proposed framework by performing a comparison between traditional pharmacokinetic models of varying complexity, by studying the effect of ROI size.
|
||
 |
0498.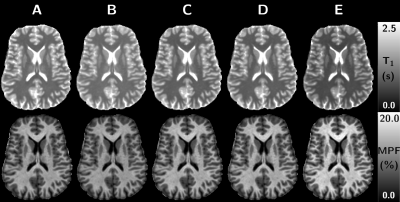 |
On the variability of single-point MPF mapping in the human brain using different Variable Flip Angle T1 mapping protocols
Lucas Soustelle1,2, Thomas Troalen3, Andreea Hertanu1,2, Maxime Guye1,2, Jean-Philippe Ranjeva1,2, Guillaume Duhamel1,2, and Olivier M. Girard1,2
1Aix Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3Siemens Healthcare SAS, Saint-Denis, France
Fast macromolecular proton fraction (MPF) mapping based on single-point (SP) quantitative MT method has shown great promises for the evaluation of myelin-related studies while allowing for acceptable scan times. The SP method requires a T1 map, which is inherently biased by magnetization transfer effects. In this work, we investigate the effect of T1 maps derived from different Variable Flip Angle (VFA) protocols on the computed MPF maps. It is shown that VFA-T1 is highly variable because of MT effects, hence biasing SP-MPF maps values. The SP-MPF methodology should therefore consider MT effects in VFA-T1 estimation, especially for cross-vendor applications.
|
|
 |
0499.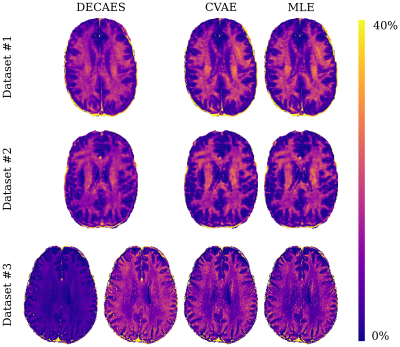 |
Rapid approximate Bayesian $$$T_2$$$ analysis under Rician noise using deep initialization
Jonathan Doucette1,2, Christian Kames1,2, Christoph Birkl3, and Alexander Rauscher1,2,4,5
1UBC MRI Research Centre, Vancouver, BC, Canada, 2Physics & Astronomy, University of British Columbia, Vancouver, BC, Canada, 3Neuroradiology, Medical University of Innsbruck, Innsbruck, Austria, 4Radiology, University of British Columbia, Vancouver, BC, Canada, 5Pediatrics, University of British Columbia, Vancouver, BC, Canada
Rapid approximate Bayesian parameter inference is investigated for $$$T_2$$$ analysis of multi spin-echo signals. We demonstrate that rapid inference using Rician noise distributions is possible using maximum likelihood estimation (MLE) if the MLE procedure is initialized with samples drawn from an approximate Bayesian posterior. This posterior is learned using a conditional variational autoencoder (CVAE) network, trained on exclusively on simulated data. Nevertheless, we show good generalization to three diverse datasets, including improved inference accuracy compared to standard nonnegative least squares-based methods which implicitly assume Gaussian noise.
|
|
0500.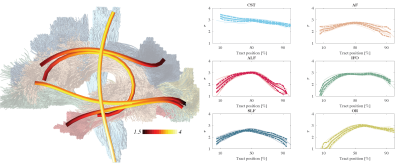 |
The variability of MR axon radii estimates in the human white matter
Jelle Veraart1, Erika P. Raven1, Luke J. Edwards2, Nikolaus Weiskopf2,3, and Derek K. Jones4,5
1Center for Biomedical Imaging, NYU Grossman School of Medicine, New York, NY, United States, 2Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3Felix Bloch Institute for Solid State Physics, Leipzig University, Leipzig, Germany, 4School of Psychology, Cardiff University, Cardiff, United Kingdom, 5Mary MacKillop Institute for Health Research, Australian Catholic University, Melbourne, Australia The accuracy of the quantification of axon radii in vivo using diffusion MRI has been promoted in recent years by hardware developments and novel biophysical modeling insights. The MR-derived effective radii are in good quantitative agreement with histology if one accounts for the intrinsic bias of diffusion MRI to larger axons. In this work, we show that the translation of MR axon diameter mapping to human neuroimaging is possible within acceptable scan times if strong diffusion-weighting gradients are available. Indeed, we demonstrate that the MR-derived effective axon radii is a reproducible and sensitive metric, with interesting inter- and along-tract variability. |
||
 |
0501.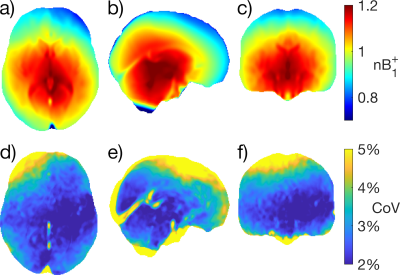 |
Characterization of B1+ Field Variation at 3 Tesla in 373 Healthy Brains over the Lifespan
Thomas MacLennan1, Peter Seres1, Julia Rickard1, Emily Stolz1, Christian Beaulieu1, and Alan H. Wilman1
1Biomedical Engineering, University of Alberta, Edmonton, AB, Canada
To perform accurate T1 and T2 mapping, $$$B_1^+$$$ maps are usually required, but not always available. For these situations, a large dataset of $$$B_1^+$$$ maps may aid in predicting $$$B_1^+$$$. In this work, Bloch-Siegert $$$B_1^+$$$ maps in the brain are characterized from a dataset of 373 healthy participants on the same 3 T. After transforming all maps to the same standard space, we show that $$$B_1^+$$$ distribution is similar across subjects with a mean CoV of 3.65% across the whole brain; slight variations were found due to brain size, shape, CSF volume, head orientation and transmit power calibration.
|
|
0502.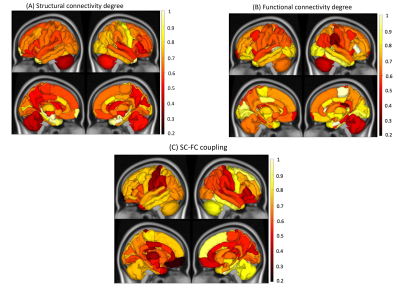 |
Predicting disability from structural and functional coupling in multiple sclerosis
Ceren Tozlu1, Keith Jamison1, Susan Gauthier1,2,3, and Amy Kuceyeski1
1Department of Radiology, Weill Cornell Medicine, New York, NY, United States, 2Judith Jaffe Multiple Sclerosis Center, Weill Cornell Medicine, New York, NY, United States, 3Department of Neurology, Weill Cornell Medicine, New York, NY, United States
The complex relationship between brain’ structural connectivity (SC) and functional connectivity (FC) has not yet been fully quantified. Previous studies have shown that an increased SC-FC coupling is associated with worse cognitive performance and higher disability in people with multiple sclerosis (pwMS). However, no study to date investigated the association of regional SC-FC coupling with disability in MS. We showed that the SC-FC coupling performed a high prediction performance in classifying pwMS by impairment level. Damage to SC, particularly in the right parsorbitalis, thalamus, and parahippocampal and left superior parietal is a hallmark of disability in MS.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.