Oral
Cancer: Contrast Agents & MRS
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 2 | 12:00 - 14:00 | Moderators: Margarida Julia-Sape & Esin Ozturk-Isik |
 |
0011.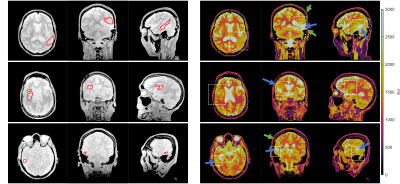 |
High-resolution T1 Mapping of High-grade Glioma
Zhibo Zhu1, Jay Acharya2, Yannick Bliesener1, R. Marc Lebel3,4, Richard Frayne3,5, and Krishna S. Nayak1,2
1Ming Hsieh Department of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 2Department of Radiology, University of Southern California, Los Angeles, CA, United States, 3Radiology and Clinical Neuroscience, Hotchkiss Brain Institute, University of Calgary, Calgray, AB, Canada, 4Global MR Applications & Workflow, GE Healthcare, Calgary, AB, Canada, 5Seaman Family MR Research Centre, Foothills Medical Centre, Calgary, AB, Canada
Native T1 mapping is necessary for quantitative DCE-MRI of brain tumor and may have independent predictive value. Previous investigations using coarse spatial resolution have found tumor to have longer T1 compared to normal white matter. In this work, we evaluate a recent millimeter-resolution whole-brain T1 mapping approach in patients with high-grade glioma. T1 values in tumor and peritumoral regions were higher than that of normal white matter, consistent with literature. We also observed T1 spatial heterogeneity in these regions, further supporting the need for high resolution pre-contrast T1 mapping for quantitative DCE-MRI.
|
|
0012.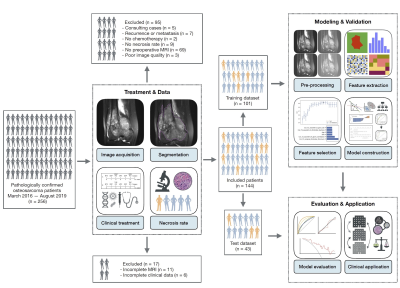 |
A nomogram combining T2WI-based radiomics features and clinical variables for prediction of neoadjuvant chemotherapy response in osteosarcoma
Chengxiu Zhang1, Jingyu Zhong2, Yangfan Hu3, Jing Zhang1, Liping Si2, Yue Xing2, Jia Geng3, Qiong Jiao4, Huizhen Zhang4, Weiwu Yao2, and Guang Yang1
1Shanghai Key Laboratory of Magnetic Resonance, East China Normal University, Shanghai, China, 2Department of Imaging, Tongren Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 3Department of Radiology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China, 4Department of Pathology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China
Osteosarcoma is the most common malignant osseous tumor and neoadjuvant chemotherapy for osteosarcoma has significantly improved survival outcomes. However, not all patients benefit from the current treatment strategy. We constructed a nomogram combined radiomics features from routinely available T2WI images and clinical variables to predict the response to neoadjuvant chemotherapy. The nomogram achieved an AUC of 0.838 (95% CI, 0.700-0.958) and DCA suggested that it has the potential to be used for preoperational prediction of pathological NAC response in osteosarcoma patients.
|
||
 |
0013.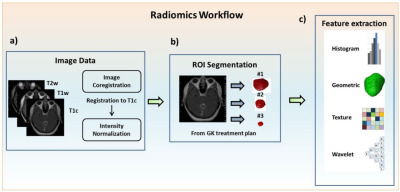 |
Using MR Radiomics to Improve Prediction of Local Tumor Control after Radiosurgery in Brain Metastases
Chien-Yi Liao1, Cheng-Chia Lee2,3,4, Huai-Che Yang2,3, Wen-Yuh Chung2,3, Hsiu-Mei Wu3,5, Wan-Yuo Guo3,5, Ren-Shyan Liu1,6,7, and Chia-Feng Lu1,8
1Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming University, Taipei, Taiwan, Taipei, Taiwan, 2Department of Neurosurgery, Neurological Institute, Taipei Veteran General Hospital, Taipei, Taiwan, Taipei, Taiwan, 3School of Medicine, National Yang-Ming University, Taipei, Taiwan, Taipei, Taiwan, 4Brain Research Center, National Yang-Ming University, Taipei, Taiwan, Taipei, Taiwan, 5Department of Radiology, Taipei Veteran General Hospital, Taipei, Taiwan, Taipei, Taiwan, 6Department of Medical Imaging, Cheng-Hsin General Hospital, Taipei, Taiwan, Taipei, Taiwan, 7Molecular and Genetic Imaging Core, Taiwan Animal Consortium, Taipei, Taiwan, Taipei, Taiwan, 8Institute of Biophotonics, National Yang-Ming University, Taipei, Taiwan, Taipei, Taiwan
Patients with non-small cell lung cancer have a high probability to develop brain metastasis during the course of the disease. The prediction of treatment response after Gamma Knife stereotactic radiosurgery (GKRS) can benefit patient management. In addition to the clinically available information (Karnofsky performance status, number of tumors, tumor volume, and primary tumor control), we proposed an MR radiomics approach to provide added values to predict the local tumor control after GKRS. We suggested that imaging characteristics extracted from preradiosurgical MRIs combined with clinical information can effectively predict local tumor control.
|
|
0014.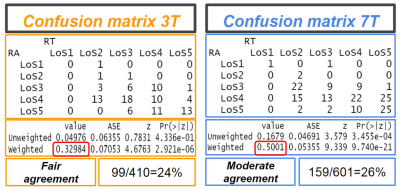 |
The power of field strength: a direct comparison of USPIO-enhanced MRI at 3 and 7T to detect suspicious lymph nodes in patients with prostate cancer
Ansje Fortuin1,2, Sjaak van Asten1, Andor Veltien1, Bart Philips1, Thomas Hambrock1, Stephan Orzada3,4, Harald Quick3,5, Jelle Barentsz1, Marnix Maas1, and Tom Scheenen1,3
1Radboudumc, Nijmegen, Netherlands, 2Radiology, Ziekenhuis Gelderse Vallei, Ede, Netherlands, 3Erwin L Hahn Institute for MR Imaging, Essen, Germany, 4University of Heidelberg, Heidelberg, Germany, 5University of Duisburg-Essen, Essen, Germany
Lymph node metastases in prostate cancer patients are mainly found in normal sized lymph nodes and detection is a major challenge. USPIO-enhanced MRI with ferumoxtran-10 discriminates between normal and suspicious lymph nodes. We examined 20 prostate cancer patients with high risk of advanced disease with USPIO-enhanced MRI at 3 and at 7 Tesla, and compared the amount, the level of suspicion, and the size of lymph nodes with two readers. More, but on average not larger suspicious nodes were annotated on 7T versus 3T MRI by both readers, with less interobserver variability at 7T.
|
||
 |
0015.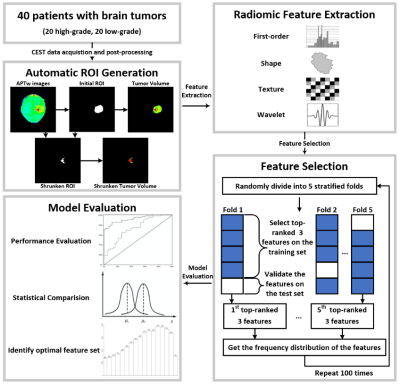 |
Radiomics-based CEST image analysis for improved performance of brain tumor grading
Jibin Tang1, Hongxi Zhang2, Zhipeng Shen3, Wenqi Wang1, Xingwang Yong1, Junjie Wen1, Xinchun Chen2, Fengyu Tian2, Weibo Chen4, Dan Wu1, and Yi Zhang1
1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, Zhejiang, China, 2Department of Radiology, Children’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China, 3Department of Neurosurgery, Children’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China, 4Philips Healthcare, Shanghai, China
CEST imaging can detect proteins and metabolites in vivo and has been successfully applied to brain tumor grading. In this work, we implemented a radiomic analysis of the APTw images, which were acquired from 40 patients with 20 confirmed high-grade brain tumors and 20 confirmed low-grade tumors. We established predictive models, assessed their performance, and compared them with conventional average APTw image intensities. The average sensitivity and AUC of the selected radiomic feature models for tumor grading were significantly higher than that of conventional mean APTw signals, demonstrating the advantage of radiomics for diagnosing brain tumors.
|
|
0016.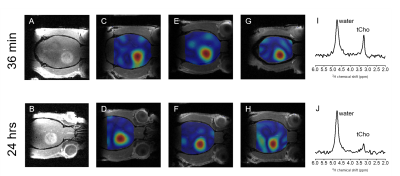 |
Delayed mapping of 2H-labeled choline using Deuterium Metabolic Imaging (DMI) reveals active choline metabolism in rat glioblastoma.
Henk M. De Feyter1, Monique A. Thomas1, Kevan L. Ip1, Kevin L. Behar2, and Robin A. de Graaf3,4
1Department of Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Department of Psychiatry, Yale University, New Haven, CT, United States, 3Department of Radiology and Biomedical Imaging, Yale University, NEW HAVEN, CT, United States, 4Department of Biomedical Engineering, Yale University, New Haven, CT, United States
Previously we reported high tumor uptake of exogenous choline in rodent brain tumor models when using Deuterium Metabolic Imaging (DMI) during intravenous infusion of [2H9]-choline. The small differences in chemical shifts of [2H9]-labeled choline, phosphocholine, glycerophosphocholine and betaine exclude accurate peak assignment, and therefore it is unclear whether blood-borne choline is metabolized intracellularly. Using different 2H-labeling strategies of choline and high resolution 2H NMR in tumor tissue extracts we identified the choline-containing metabolites observed during intravenous infusion, as well as after 24 hrs.
|
||
0017.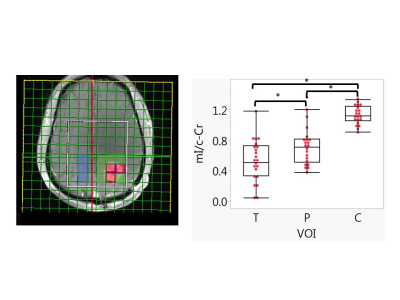 |
Predictive Value of Myo-inositol Measured by MRSI during Anti-angiogenic Treatment in Recurrent Glioblastoma
Michael Wenke1, Jorg Dietrich2, Elizabeth Gerstner2, Otto Rapalino3, Julian He3, Daniel Kim1, Melanie Fu1, Pratik Talati4, Mohamed El Abtah1, Anna Vaynrub1, Sharif Natheir1, Mark Vangel3, Isabel Arrillaga-Romany2, Forst
Deborah2, Yi-Fen Yen1, Ovidiu Andronesi1, Jayashree Kalpathy-Cramer1, Tracy Batchelor5, Bruce Rosen1, R. Gilberto Gonzalez3, and Eva-Maria Ratai1
1Radiology / Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Neurology / Cancer Center, Massachusetts General Hospital, Boston, MA, United States, 3Radiology, Massachusetts General Hospital, Boston, MA, United States, 4Neurosurgery, Massachusetts General Hospital, Boston, MA, United States, 5Neurology, Brigham and Women's Hospital, Boston, MA, United States
Patients with recurrent glioblastoma (rGBM) are commonly treated with anti-angiogenic agents such as bevacizumab (BEV), but not all benefit from this therapy. We examined whether MR spectroscopic imaging (MRSI) of myo-inositol (mI) could distinguish short-term survivors from longer term survivors (>9 month). We scanned twenty-two rGBM patients with MRSI at baseline prior to bevacizumab-based therapy, as well as 1-2 days, 4 weeks, 6-8 weeks and 16 weeks after treatment. We found that low tumoral myo-inositol normalized by creatine (Cr) on the contralateral site (mI/c-Cr) prior to and during anti-angiogenic therapy is predictive of poor survival.
|
||
 |
0018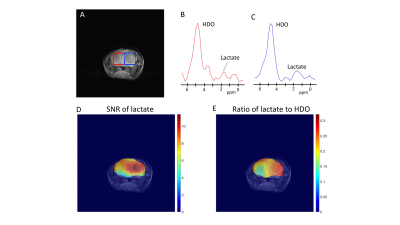 |
Deuterium magnetic resonance spectroscopy using 2H-pyruvate allows non-invasive in vivo imaging of TERT expression in brain tumors Video Permission Withheld
Georgios Batsios1, Celine Taglang1, Meryssa Tran1, Anne Marie Gillespie1, Joseph Costello2, Sabrina Ronen1, and Pavithra Viswanath1
1Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 2Neurological Surgery, UCSF, San Francisco, CA, United States
Telomerase reverse transcriptase (TERT) expression is essential for tumor proliferation. Since TERT is exclusively expressed in tumor cells, TERT is also an attractive therapeutic target. However, non-invasive methods of imaging TERT are lacking. Here, we show that TERT expression in preclinical patient-derived brain tumor models is associated with elevated steady-state levels of NADH, an effect that can be non-invasively visualized in vivo by deuterium metabolic imaging using [U-2H]pyruvate. Since 2H-MRS can be readily implemented on clinical MR scanners, our results provide an innovative, clinically translatable method of integrating information regarding a fundamental cancer hallmark, i.e. TERT, into glioma patient management.
|
|
0019.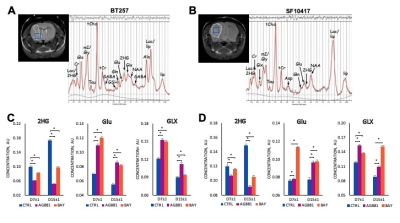 |
Early noninvasive metabolic biomarkers of mutant IDH inhibition in low-grade glioma models
Marina Radoul1, Donghyun Hong1, Anne Marie Gillespie1, Chloé Najac1, Pavithra Viswanath1, Russell O. Pieper2,3, Joseph Costello2, H. Artee Luchman4, and Sabrina M. Ronen1,3
1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 2Neurological Surgery UCSF Weill Institute for Neurosciences, University of California San Francisco, San Francisco, CA, United States, 3Brain Tumor Research Center, University of California San Francisco, San Francisco, CA, United States, 4Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
Targeting mutant IDH, which is present in ~80% of glioma, is being tested as a new therapeutic approach. In the current study, we investigated metabolic alterations in response to mutant IDH inhibition by either AG-881 or BAY-1436032 in orthotopic patient-derived glioma models. Using high resolution in vivo 1H MRS we detected, in addition to a decrease in 2HG, an early increase in glutamate and the combined glutamine/glutamate signals. These were associated with slowdown of tumor growth and ultimately longer animal survival. This identifies potential early metabolic biomarkers of glioma response to mutant IDH inhibition.
|
||
 |
0020.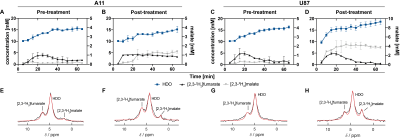 |
Imaging response to radio-chemotherapy in brain tumor models using [2,3-2H2]fumarate and deuterium magnetic resonance spectroscopic imaging
Friederike Hesse1, Alan Wright1, Vencel Somai1,2, Flaviu Bulat1,3, and Kevin Brindle1,4
1Cancer Research UK Cambridge Institute, Cambridge, United Kingdom, 2Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 3Department of Chemistry, University of Cambridge, Cambridge, United Kingdom, 4Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom
Metabolic imaging of brain tumor responses to radio-chemotherapy can give an early indication of treatment outcome. We show here that Deuterium Metabolic Imaging (DMI) with 2H-labeled fumarate can be used to detect early evidence of cell death following radio-chemotherapy in an orthotopic patient-derived glioblastoma model. 2H spectra were acquired from tumors, following an injection of 2H-labeled fumarate. Within one week of treatment the rate of tumor malate production increased significantly. Increased levels of labeled malate were also evident in spectroscopic images of the tumors. These measurements were compared with 13C MRSI measurements of [1,4-13C2]malate production from hyperpolarized [1,4-13C2]fumarate1.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.