Oral
Young Investigator Awards Presentations
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 8 | 14:00 - 16:00 | Moderators: |
0113.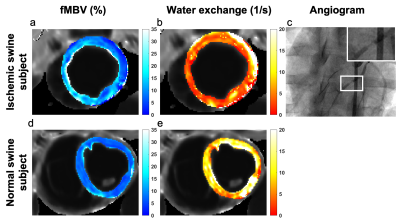 |
Estimation of fractional myocardial blood volume and water exchange using ferumoxytol-enhanced MRI
Caroline Colbert1, Michael A. Thomas2, Ran Yan3, Aleksandra Radjenovic4, J. Paul Finn1,5, Peng Hu1,3,5, and Kim-Lien Nguyen1,2,5
1Physics and Biology in Medicine Graduate Program, David Geffen School of Medicine at UCLA, Los Angeles, CA, United States, 2Division of Cardiology, UCLA David Geffen School of Medicine, Los Angeles, CA, United States, 3Department of Radiology, UCLA David Geffen School of Medicine, Los Angeles, CA, United States, 4Institute of Cardiovascular & Medical Sciences, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, United Kingdom, 5Diagnostic Cardiovascular Imaging Laboratory, Department of Radiological Sciences, UCLA David Geffen School of Medicine, Los Angeles, CA, United States
We optimized and tested a two-compartment water exchange model for fractional myocardial blood volume (fMBV) quantification. Nine healthy swine and one swine model with single-vessel coronary stenosis underwent MOLLI T1 imaging at 3.0 T following multiple individual ferumoxytol infusions. Healthy normal swine showed a mean mid-ventricular fMBV of 7.2 ± 1.4% and water exchange rate of 11.3 ± 5.1 s-1. In one swine model with artificially‑induced single-vessel coronary stenosis, quantitative pixel-wise fMBV showed regional differences in hypoperfused relative to perfused regions. This study demonstrates the feasibility of fMBV estimation using multi-dose ferumoxytol‑enhanced MRI with a two-compartment water exchange model.
|
||
0114. |
Brain oxygen extraction is differentially altered by Alzheimer’s and vascular diseases
Dengrong Jiang1,2, Zixuan Lin1,2, Peiying Liu1, Sandeepa Sur1, Cuimei Xu1, Kaisha Hazel1, George Pottanat1, Jacqueline Darrow3, Jay J. Pillai1,4, Sevil Yasar5, Paul Rosenberg6, Abhay Moghekar3, Marilyn Albert3, and Hanzhang
Lu1,2,7
1The Russell H. Morgan Department of Radiology & Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Department of Biomedical Engineering, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 6Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 7F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Research Institute, Baltimore, MD, United States
Alzheimer’s disease, vascular cognitive impairment and their concurrence represent the most common types of cognitive dysfunction. There exists a considerable overlap in their clinical symptoms and neuroimaging features, and we still lack effective tools for their differential diagnosis. This work demonstrated that cerebral oxygen-extraction-fraction (OEF) was differentially affected by Alzheimer’s (decrease OEF) and vascular (increase OEF) pathology. In individuals with low vascular risks, lower OEF was associated with worse cognitive performance and greater amyloid burden. In impaired patients, higher OEF was associated with great vascular risk factors. These findings suggest OEF can be useful in etiology-based diagnosis of cognitive impairment.
|
||
0115.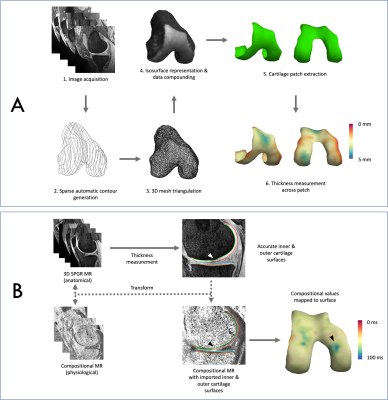 |
Three-Dimensional Surface-Based Analysis of Cartilage MRI Data in Knee Osteoarthritis: Validation and Initial Clinical Application
James W. MacKay1,2, Joshua Kaggie1, Graham M. Treece3, Stephen M. McDonnell4, Wasim Khan4, Alexandra R. Roberts5,6, Rob L. Janiczek5, Martin J. Graves1, Tom D. Turmezei2,7, Andrew W. McCaskie4, and Fiona J. Gilbert1
1Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 2Norwich Medical School, University of East Anglia, Norwich, United Kingdom, 3Department of Engineering, University of Cambridge, Cambridge, United Kingdom, 4Department of Surgery, University of Cambridge, Cambridge, United Kingdom, 5Clinical Imaging, GlaxoSmithKline, London, United Kingdom, 6Antaros Medical, Uppsala, Sweden, 7Department of Radiology, Norfolk & Norwich University Hospital, Norwich, United Kingdom Conventional MRI outcome measures for cartilage in knee osteoarthritis (OA) clinical studies lack responsiveness and require time-consuming manual analysis. Here we validate and clinically implement a semiautomatic surface-based approach termed 3D Cartilage Surface Mapping (3D-CaSM) which overcomes these issues. Validation data demonstrate comparable bias, precision, repeatability and reproducibility to expert manual segmentation (current standard) but with >10 fold reduction in analysis time. Clinical data indicate improved sensitivity to change in one observational and two interventional (exercise, knee joint distraction) studies.
|
||
0116.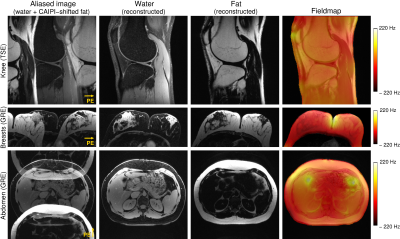 |
Simultaneous Multiple Resonance Frequency Imaging (SMURF): Fat‑water imaging using multi‑band principles
Beata Bachrata1,2, Bernhard Strasser1,3, Wolfgang Bogner1, Albrecht Ingo Schmid4, Radim Korinek5, Martin Krššák1,2,6, Siegfried Trattnig1,2, and Simon Daniel Robinson1,7,8
1High Field MR Centre, Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna, Austria, 2Karl Landsteiner Institute for Clinical Molecular MR in Musculoskeletal Imaging, Vienna, Austria, 3Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 4High Field MR Centre, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 5Institute of Scientific Instruments of the CAS, Brno, Czech Republic, 6Department of Internal Medicine III, Division of Endocrinology and Metabolism, Medical University of Vienna, Vienna, Austria, 7Centre of Advanced Imaging, University of Queensland, St. Lucia, Australia, 8Department of Neurology, Medical University of Graz, Graz, Austria
Imaging of body regions containing a significant amount of fat is adversely affected by chemical shift artefacts. We propose a new fat-water imaging method that uses spectrally selective dual-band excitation and CAIPIRINHA to generate separate images of fat and water simultaneously as well as chemical shift-corrected, recombined fat-water images. Gradient-echo and turbo spin-echo variants of this Simultaneous Multiple Resonance Frequency Imaging (SMURF) approach yielded fat-water separation which was similar to or better than state-of-the-art techniques in the knee, breasts and abdomen and generated recombined fat-water images in which chemical shift effects were fully eliminated.
|
||
0117.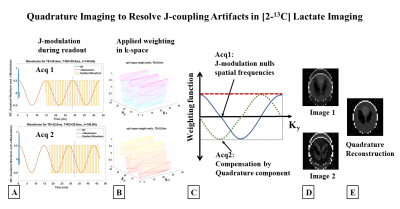 |
MRI of [2-13C]Lactate without J-coupling artifacts
Keshav Datta1 and Daniel Spielman1
1Department of Radiology, Stanford University, Stanford, CA, United States
Metabolic imaging using hyperpolarized [2-13C]Pyruvate has the potential to simultaneously probe glycolysis and Kreb’s cycle, but one of its major limitations is the difficulty in imaging [2-13C]Lactate. The peak-splitting induced by the J-coupling between the C2 carbon and its attached proton causes ghosting and blurring artifacts, depending on the k-space trajectory. We propose two novel techniques, the first a two-shot approach combining in-phase and quadrature images acquired at echo times differing by 1/2J and the second a single-shot method employing a highly narrowband radiofrequency excitation pulse that images a single peak from the doublet, to resolve the J-modulated artifacts.
|
||
0118.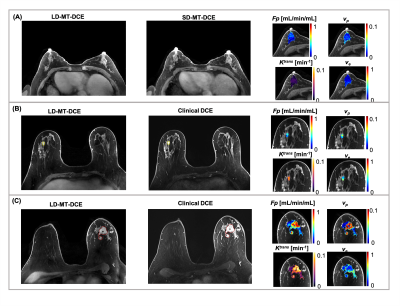 |
Five-Dimensional Quantitative Low-Dose Multitasking Dynamic Contrast- Enhanced MRI (LD-MT-DCE): Preliminary Study on Breast Cancer
Nan Wang1,2, Yibin Xie1, Zhaoyang Fan1,2, Sen Ma1,2, Rola Saouaf3, Yu Guo1,4, Stephen L. Shiao5,6, Anthony G. Christodoulou1,2, and Debiao Li1,2
1Biomedical Imaging Research Institute, Cedars Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 3Department of Imaging, Cedars Sinai Medical Center, Los Angeles, CA, United States, 4Department of Radiology, Tianjin First Central Hospital, Tianjin, China, 5Department of Radiation Oncology, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 6Biomedical Sciences, Division of Immunology, Cedars-Sinai Medical Center, Los Angeles, CA, United States
DCE MRI is an important technique for diagnosing breast cancer, but continues to face technical challenges and gadolinium deposition concerns. In this work, we proposed a low-dose Multitasking DCE (LD-MT-DCE) technique, enabling dynamic-T1-mapping based quantitative characterization of tumor blood flow and vascular properties with whole-breast coverage, a spatial resolution of 0.9×0.9×1.1mm3, and a temporal resolution of 1.4 s using only 20% gadolinium dose. An in vivo study showed excellent image quality and repeatability (ICC≥0.99) for LD-MT-DCE and consistent diagnosis to standard-dose clinical DCE. The kinetic parameters showed significant differences between normal breast tissue, and benign and malignant tumors.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.