Shir Filo1, Rona Shaharabani1, and Aviv Mezer1
1The Edmond and Lily Safra Center for Brain Science, The Hebrew University of Jerusalem, Jerusalem, Israel
1The Edmond and Lily Safra Center for Brain Science, The Hebrew University of Jerusalem, Jerusalem, Israel
We propose an
in vivo quantitative MRI approach for assessment of iron forms, based on
the dependency of R1 on R2*. Our method was established
in phantoms and validated against histology. It predicts the heterogeneous
distribution of iron-binding proteins with age and across the in-vivo brain.
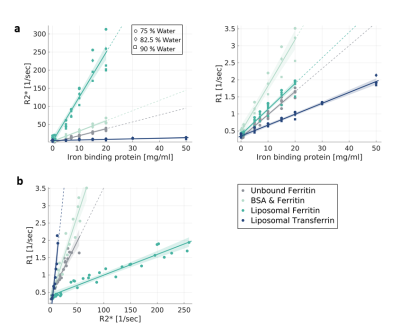
Establishing the iron relaxivity framework
in vitro. (a) R2* and R1 vary with the concentration
of iron-binding proteins (x-axis), with their
type (transferrin, ferritin) and with the molecular environment (liposomes,
saline, BSA) (different colors). Data points are phantom samples’
medians. Symbols represent water fractions. (b) The
dependency of R1 on R2* for different molecular compounds and environments of iron.
R2* and R1 of samples with varying water and protein concentrations were binned, data points
are the bins’ median. The R1-R2* slopes are
different for each iron form.
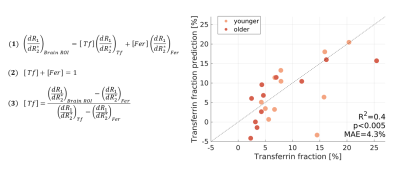
Fully-constrained model predicts the
fractions of iron-binding proteins in the in vivo human brain. The measured R1-R2* slope in each brain
area was modeled (Eq. 1) as a weighted sum of the R1-R2* slopes of transferrin
(Tf) and ferritin (Fer) (estimated in liposomal phantoms). The Tf
and Fer fractions sum to one (Eq. 2). Rearranging the model (Eq. 3) allows
to predict the transferrin fraction (Tf/(Tf+Fer), y-axis) for
younger (<64) and older subjects in
11 brain regions. There are no free parameters. In the x-axis is the Tf fraction
measured post-mortem5,6,9,10. MAE=mean absolute error.
