Yohann Mathieu-Daudé1, Mélissa Vincent1, Julien Valette1, and Julien Flament1
1Université Paris-Saclay, Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), Centre National de la Recherche Scientifique (CNRS), Molecular Imaging Research Center (MIRCen), Laboratoire des Maladies Neurodégénératives, Fontenay-aux-Roses, France
1Université Paris-Saclay, Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), Centre National de la Recherche Scientifique (CNRS), Molecular Imaging Research Center (MIRCen), Laboratoire des Maladies Neurodégénératives, Fontenay-aux-Roses, France
In this study, we
investigated the compartmental origin of glucoCEST signal using glucose
analogues. We found that intravascular does not contribute to CEST effect.
However, extravascular/extracellular and intracellular spaces both do.
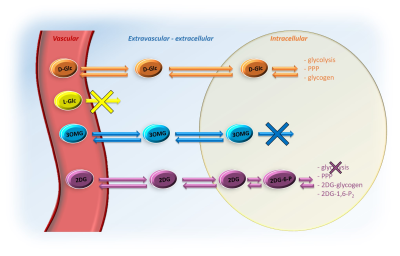
Figure 1: Schema of transport and degradation of D-Glc, L-Glc, 3OMG and 2DG
After injection into the vascular space, molecules of interest (except L-Glc) are transported into the extravascular/extracellular space, then into the intracellular space by GLUTs transporters. D-Glc is transported in cells where it can be metabolized through glycolysis, penthose phosphate pathway (PPP) or into glycogen. 3OMG is transported in cells but does not undergo further metabolism. 2DG is transported in cells where it metabolized into 2DG6P by hexokinase but it does not undergo glycolysis.
After injection into the vascular space, molecules of interest (except L-Glc) are transported into the extravascular/extracellular space, then into the intracellular space by GLUTs transporters. D-Glc is transported in cells where it can be metabolized through glycolysis, penthose phosphate pathway (PPP) or into glycogen. 3OMG is transported in cells but does not undergo further metabolism. 2DG is transported in cells where it metabolized into 2DG6P by hexokinase but it does not undergo glycolysis.
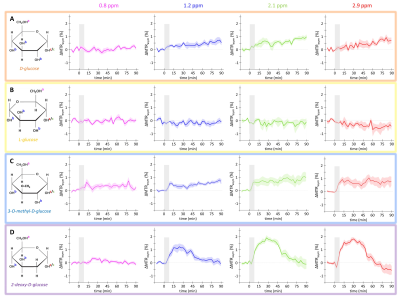
Figure 3: in vivo kinetics of glucoCEST signal
Intravenous injection of D-Glc (A), L-Glc (B), 3OMG (C) and 2DG (D). GlucoCEST variations were measured before and after intravenous injection (gray area) at 0.8 ppm, 1.2
ppm, 2.1 ppm, 2.9 ppm. Mean ± SEM variations of CEST signal compared to baseline are shown (n=5, 3, 9 and 7 respectively).