Antonia Kaiser1, Marco A. Bottelier1,2, Michelle M. Solleveld1, Hyke G.H. Tamminga1,3, Cheima Bouziane1, Ramon J.L. Lindauer4,5, Paul J. Lucassen6, Michiel B. de Ruiter1,7, Anouk Schrantee1, and Liesbeth Reneman1
1Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 2Child Study Center, Accare, Groningen, Netherlands, 3Dutch Autism and ADHD research center, University of Amsterdam, Amsterdam, Netherlands, 4Department of Child and Adolescent Psychiatry, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 5De Bascule, Academic Centre for Child and Adolescent Psychiatry, Amsterdam, Netherlands, 6Brain Plasticity Group, Swammerdam Institute for Life Sciences, Center for Neuroscience, University of Amsterdam, Amsterdam, Netherlands, 7Division of Psychosocial Research and Epidemiology, Netherlands Cancer Institute, Amsterdam, Netherlands
1Department of Radiology and Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 2Child Study Center, Accare, Groningen, Netherlands, 3Dutch Autism and ADHD research center, University of Amsterdam, Amsterdam, Netherlands, 4Department of Child and Adolescent Psychiatry, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 5De Bascule, Academic Centre for Child and Adolescent Psychiatry, Amsterdam, Netherlands, 6Brain Plasticity Group, Swammerdam Institute for Life Sciences, Center for Neuroscience, University of Amsterdam, Amsterdam, Netherlands, 7Division of Psychosocial Research and Epidemiology, Netherlands Cancer Institute, Amsterdam, Netherlands
We report in this randomized
clinical trial, that although depressive and anxiety symptoms at baseline
negatively predicted ADHD symptom change in adults, age-dependent effects on
amygdala reactivity were absent.
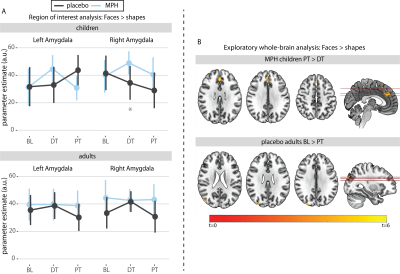
Figure
3.| FMRI results of the face-matching task. A) Region of interest
analysis: Post-hoc tests showed significant differences between
conditions in right amygdala reactivity for children at 8 weeks (DT) (mean with
95% CI). B) Exploratory whole-brain analysis: Increased reactivity in the
Superior-Frontal-Gyrus and Paracingulate-Cortex in methylphenidate treated
children from DT to PT and decreased reactivity in the Lateral-Occipital-Cortex
in placebo-treated adults from BL to PT.
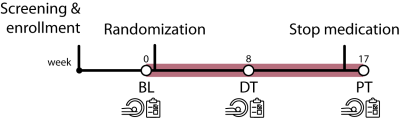
Figure 1.| Timeline of the ePOD-MPH RCT. A 16-week double-blind, randomized, placebo-controlled, multicenter trial with methylphenidate and a blinded endpoint evaluation in stimulant treatment-naive patients with ADHD. We measured fMRI activity on a face-matching task at three times points (baseline (BL), eight weeks during treatment (DT), and one week after the trial (post-treatment (PT)). Furthermore, clinical measures of anxiety, depression, and emotional lability were assessed.
