Ken Sakaie1, Janel Fedler2, Jon Yankey2, Kunio Nakamura1, Josef Debbins3, Mark J. Lowe1, Paola Raska1, and Robert J. Fox1
1The Cleveland Clinic, Cleveland, OH, United States, 2University of Iowa, Iowa City, IA, United States, 3Barrow Neurological Institute, Phoenix, AZ, United States
1The Cleveland Clinic, Cleveland, OH, United States, 2University of Iowa, Iowa City, IA, United States, 3Barrow Neurological Institute, Phoenix, AZ, United States
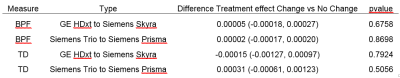
We find that accounting for the specific type of hardware change is optimal, but trends differ between different imaging measures (BPF and TD). We expect these results to be useful in planning future clinical trials.

Table 2. Effect of adjusting for hardware changes. BPF is unitless, TD units are 10-3 mm2/sec. Values are change per year, with 95% CI’s in parentheses. Models are: no adjustment for hardware changes (Original), data acquired after a hardware change excluded (Exclude), hardware change treated as a binary yes/no time-dependent covariate (Binary) and type of hardware change is a time-dependent covariate (Type). Lower AIC indicates a better fit, with a change of 2 being substantial.