Or Perlman1, Bo Zhu1,2, Moritz Zaiss3,4, Naoyuki Shono5, Hiroshi Nakashima5, E. Antonio Chiocca5, Matthew S. Rosen1,2, and Christian T. Farrar1
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Department of Physics, Harvard University, Cambridge, MA, United States, 3Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 4Department of Neuroradiology, University Clinic Erlangen, Erlangen, Germany, 5Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Department of Physics, Harvard University, Cambridge, MA, United States, 3Magnetic Resonance Center, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 4Department of Neuroradiology, University Clinic Erlangen, Erlangen, Germany, 5Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States
A machine-learning framework was
expanded
for simultaneously designing the optimal CEST protocol
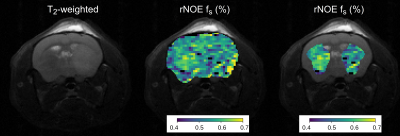
and extracting fully quantitative maps in-vivo. A mouse tumor
rNOE volume-fraction was significantly decreased, in agreement with
previous human studies.
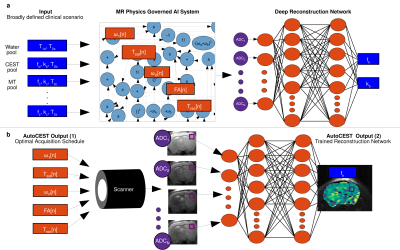
Fig. 1 AutoCEST pipeline. a.
Pre-experiment step. The broad expected range of properties (blue
rectangles) are given as input to an MR physics governed AI system,
which dynamically optimizes the protocol parameters (orange
rectangles). The resulting “ADC” MR signals are then decoded into
quantitative parameters using a deep
reconstruction network. b.
Experiment step. The optimal schedule parameters are loaded into the
scanner, resulting in a set of N raw images. The resulting images are
fed voxelwise into the trained reconstruction network, resulting in
quantitative CEST maps.