Jia Xu1, Rolf F. Schulte2, William R. Kearney1, and Vincent A. Magnotta1,3,4
1Radiology, University of Iowa, Iowa City, IA, United States, 2GE Global Research, Munich, Germany, 3Psychiatry, University of Iowa, Iowa City, IA, United States, 4Biomedical Engineering, University of Iowa, Iowa City, IA, United States
1Radiology, University of Iowa, Iowa City, IA, United States, 2GE Global Research, Munich, Germany, 3Psychiatry, University of Iowa, Iowa City, IA, United States, 4Biomedical Engineering, University of Iowa, Iowa City, IA, United States
The peak integration by
simple summation of the magnitude spectrum is the most sensitive and robust 31P
MRS quantification method to detect small ATP concentration changes. The
test-retest repeatability of in-vivo ATP quantification is less than 3%.
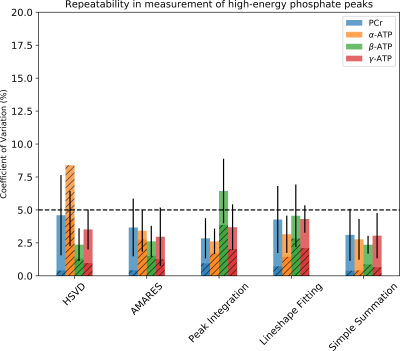
Figure 3. Interscan test-retest repeatability of PCr, α-ATP, β-ATP, and γ-ATP
peaks from quantitative, non-localized 31P MRS of 7 human subjects.
The corresponding CVs estimated by Monte Carlo Simulation are shown in hatched
bars. The dashed horizon line represents a 5% threshold.
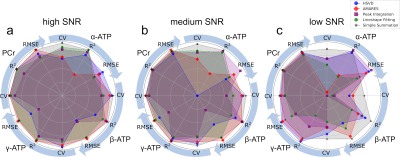
Figure 2. Comparison of Trueness
(RMSE), linearity (R2), and relative uncertainty (CV) of α-ATP,
β-ATP, γ-ATP, and PCr peaks quantified by HSVD, AMARES, conventional peak
integration, lineshape fitting, and simple summation. The RMSE, R2, and CV values
are normalized to (0, 1) range so that the reference point (center) represents
the worst performance (0) and the circle represents the best performance (1).
The normalized RMSE, R2, and CV values of α-ATP, β-ATP, γ-ATP, and
PCr peaks are distributed evenly along the angular axes (circle).