Naila Rahman1,2, Kathy Xu2, Nico Arezza1,2, Kevin Borsos1,2, Matthew Budde3, Arthur Brown2,4, and Corey Baron1,2
1Medical Biophysics, Western University, London, ON, Canada, 2Robarts Research Institute, London, ON, Canada, 3Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 4Anatomy and Cell Biology, Western University, London, ON, Canada
1Medical Biophysics, Western University, London, ON, Canada, 2Robarts Research Institute, London, ON, Canada, 3Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 4Anatomy and Cell Biology, Western University, London, ON, Canada
In a rodent
traumatic brain injury model, changes in mean diffusivity dependence
on oscillating gradient frequency (sensitive to structural disorder such as beading)
and spherical tensor kurtosis (sensitive to size heterogeneity) were
observed, compared to healthy mice.
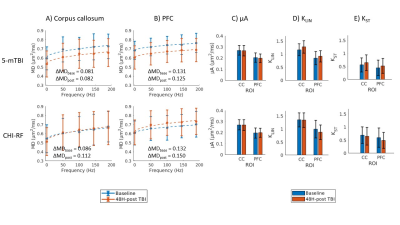
Figure 4. Preliminary data (one mouse per row).
MD dependence on frequency is shown 48H-post 5-mTBI and 48H-post
CHI-RF in A) the corpus callosum and B) the PFC. All error bars
represent the standard deviation in the ROI (in one mouse) and the
dotted lines are the least square fits to frequency0.5.
ΔMDbase
(ΔMD at baseline) and ΔMDpost
(ΔMD post-TBI) are reported for each case.
Baseline and 48H-post TBI data are
also shown for µA (C), KLIN (D), and KST (E)
in the corpus callosum (CC) and PFC.
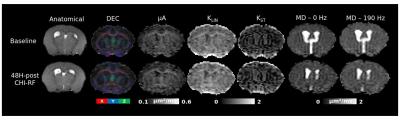
Figure
3. Parameter maps from the same mouse taken at baseline and 48H-post
CHI-RF. The direction-encoded color (DEC) maps were acquired using
the b = 2000 s/mm2 linear acquisitions from the µA
protocol. Microscopic anisotropy (µA), linear kurtosis (KLIN),
and spherical tensor kurtosis (KST) were estimated from
the µA protocol. From the OGSE protocol, mean diffusivity (MD) maps
are shown at 0 Hz and 190 Hz.
