Shruti Agarwal1, Jay J. Pillai1,2, and Hanzhang Lu3,4
1Division of Neuroradiology, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Division of MR Research, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
1Division of Neuroradiology, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3Division of MR Research, Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 4F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States
Areas of NAWM that develop WMH over time have reduced CBF at baseline
and these abnormalities progress faster than structural properties such as
diffusion or T1. CBF may provide an early marker for progression of age-related
WM disease.
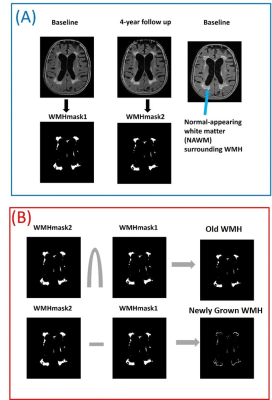
Figure
1: (A): White
matter hyperintense (WMH) regions are automatically segmented on FLAIR images
from baseline and follow-up and named as WMHmask1
and WMHmask2. Blue arrow shows the normal appearing white matter (NAWM) that
progressed to WMH in 4-year follow up. (B):
The intersection of WMHMask1 & WMHMask2 is referred as “Old WMH” at
baseline and the subtraction of WMHMask1 from WMHMask2 [(WMHMask2-WMHMask1)>0]
is referred as “Newly Grown WMH” at 4-year follow up (which was NAWM at
baseline).
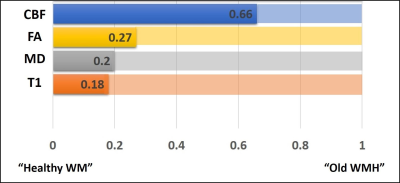
Figure 4: The relative values of diffusion,
perfusion, and T1 signal intensities among the three tissue types is displayed.
The bars are displayed such that the “Healthy WM” is on the left-end, the “Old
WMH” is displayed on the right-end, with the “Newly Grown WMH” displayed
proportionally along the bar. It can be seen that the characteristics of the
“Newly Grown WMH” are closer to the healthy tissue in all structural/anatomical
parameters, but their CBF resembles more to the “Old WMH” tissues.