Marina Celestine1, Jean-Baptiste Pérot1, Muriel Jacquier-sarlin2, Karine Cambon1, Julien Flament1, Alain Buisson2, Anne-Sophie Hérard1, and Marc Dhenain1
1Université Paris-Saclay, Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), Centre National de la Recherche Scientifique (CNRS), Molecular Imaging Research Center (MIRCen), Laboratoire des Maladies Neurodégénératives, Fontenay-aux-roses, France, 2University Grenoble Alpes, Inserm, U1216, Grenoble Institut Neurosciences (GIN), Grenoble, France
1Université Paris-Saclay, Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), Centre National de la Recherche Scientifique (CNRS), Molecular Imaging Research Center (MIRCen), Laboratoire des Maladies Neurodégénératives, Fontenay-aux-roses, France, 2University Grenoble Alpes, Inserm, U1216, Grenoble Institut Neurosciences (GIN), Grenoble, France
Exposition to Alzheimer's disease related-Aβ variant lead to memory
impairment, brain connectivity alteration and decreased brain glutamate
levels in transgenic mouse model.
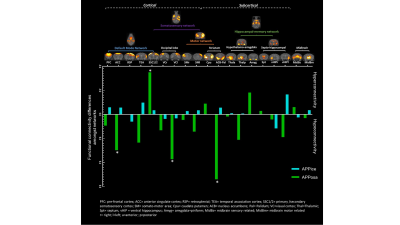
Figure 2. Aβosa and Aβice induce seed-based
analysis of connectivity changes amongst network. Whole-brain dictionary learning
maps depicting 19 cortical and subcortical components found in 4 main networks (upper).
Group differences seed-based connectivity pattern through components for
inoculum site (dentate gyrus)(lower). Asterisk represents significant p-value (p<0.001) of the
group difference in Fisher z-transformed correlation.
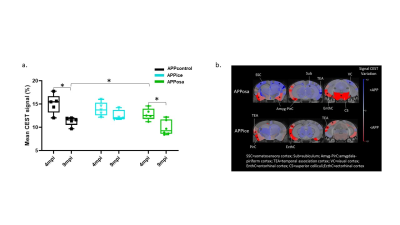
Figure 5. Decreased glutamate levels after Aβosa inoculation in APPswe/PS1de9
mice. a. GluCEST average signal
evolution in APPice, APPosa and APPcontrol mice shows age-related modification
detected in 9 mpi animals. They are rescued by Aβice inoculation. b. Variation maps of GluCEST at 4mpi (right). Variations was
calculated in every region from the atlas. Colors represent hyposignal (blue)
and hypersignal (red) comparing to control mice (*p<0.05).