Rui Vasco Simoes1, Rafael N Henriques1, Beatriz M Cardoso1, Francisca F Fernandes1, Jonas L Olesen2, Sune N Jespersen2, and Noam Shemesh1
1Champalimaud Research, Champalimaud Foundation, Lisbon, Portugal, 2Center of Functionally Integrative Neuroscience (CFIN) and MINDLab, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
1Champalimaud Research, Champalimaud Foundation, Lisbon, Portugal, 2Center of Functionally Integrative Neuroscience (CFIN) and MINDLab, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
Glycolytic and oxidative turnover rates of glucose can be
measured in mouse gliomas with DGE-2H-MRS. MP-PCA denoising improves
the time-course detection and quantification of glucose oxidation, which in
turn demonstrates correlation with MRI features of heterogeneity in
the tumor region.
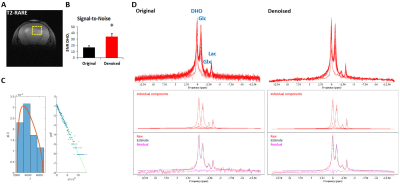
Figure 2 – Improvement of DGE 2H-MRS spectral quality upon MP-PCA
denoising. GL7: (A) T2-RARE showing the tumor region
selected for DGE 2H-MRS (yellow dashed line); (B) SNRDHOi
before and after denoising (average±SD); (C) left – eigenvalue spectrum from PCA decomposition (blue) and fit of MP distribution (orange), right – Gaussian distribution of denoising
residuals, verified by the linearity of their logarithm; (D) DGE 2H-MRS
data stacked, original and denoised, and spectral fitting including individual components, raw data,
estimate and residual. * p=0.0003, paired t-Test.
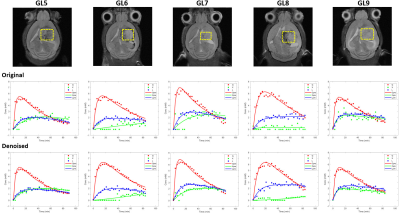
Figure 3 – Robustness of the kinetic
model for different tumors and improvement of Glx fitting with MP-PCA denoising. Tumor volumes selected for DGE 2H-MRS
(yellow dashed line) overlaid on reference T2-RARE transversal images for each
animal (GL5-9, top). Fitting of Glc (red line), Glx (green line) and Lac (blue
line) time-course changes displayed, before (center) and after denoising
(bottom).
