Yachao Liu1, Hongkai Yu2, Jiajin Liu1, Xiaojun Zhang1, Mu Lin3, Holger Schmidt4, Jiangping Gao2, and Baixuan Xu1
1Department of Nuclear Medicine, Chinese PLA General Hospital, Beijing, China, 2Department of Urology Surgery, Chinese PLA General Hospital, Beijing, China, 3MR collaborations, Diagnostic Imaging, Siemens Healthcare, Shanghai, China, 4MR Education, Customer Services, Siemens Healthcare, Erlangen, Germany
1Department of Nuclear Medicine, Chinese PLA General Hospital, Beijing, China, 2Department of Urology Surgery, Chinese PLA General Hospital, Beijing, China, 3MR collaborations, Diagnostic Imaging, Siemens Healthcare, Shanghai, China, 4MR Education, Customer Services, Siemens Healthcare, Erlangen, Germany
18F-PSMA PET/CT-US or PET/MRI-US fusion-targeted
prostate biopsy are feasible for prostate cancer diagnosis due to its
high detection rate of clinically significant prostate cancer. PET/MR can rule
out some false PET-positive lesions, which may potentially reduce unnecessary biopsies.
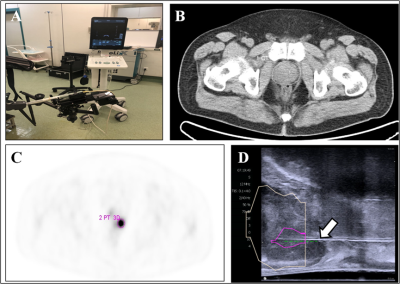
Figure 1. 18F-PSMA PET/CT-US or PET/MRI-US fusion targeted
prostate biopsy for the intraprostatic PET-positive lesions were performed (A). The
boundaries of the prostate were delineated on the CT image (B, white circle). The
PET-positive lesion was marked as the target for biopsy (C, pink circle). The previous delineated prostate and PET-positive
lesion from PET/CT was registered to the prostate volume acquired from the
3-dimensional transrectal ultrasonography; the puncture needle (D, arrow) then
reached the target biopsy area.
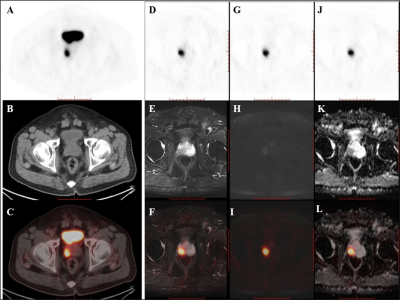
Figure 2. PET/CT
found one PET-positive lesion in the prostate gland (A: PET, B: CT, C: fused PET/CT).
PET/MRI showed short T2 signal (D: PET, E: T2WI, F: fused PET/T2WI), high DWI
signal (G: PET, H: DWI, L: fused PET/DWI), and a decreased ADC value at the
site of the PET-positive lesion (J: PET, K: ADC map, L: fused PET/ADC map). The
subsequent pathology confirmed prostate cancer.