Digital Poster
AI-Powered Analysis in Cardiovascular MRI
ISMRM & ISMRT Annual Meeting & Exhibition • 10-15 May 2025 • Honolulu, Hawai'i

 |
Computer Number: 1
4269. Assessing
Deep Learning's Ability to Segment Aortic Valve Calcifications
in Contrast-Free Cardiac MRI
E. Almar-Munoz, C. Kremser, M. Haltmeier, A. Mayr
Medical University of Innsbruck, Innsbruck, Austria
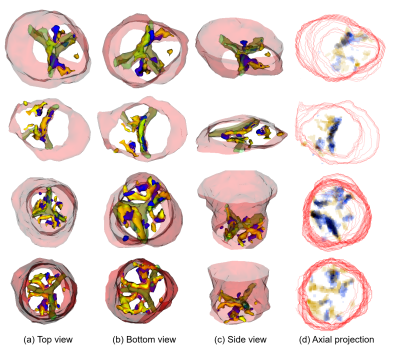
Impact: Calcifications are not visible on contrast-free
CMR to the human eye, so we tested AI segmentation using
CTA-registered labels, achieving low results (DSC 0.309).
While a larger dataset or improved registration may help,
the task's physical feasibility remains uncertain.
|
|
 |
Computer Number: 2
4270. AI-Driven
Quantification of Aortic Diameters from Contrast-Enhanced MRA of
the Thoracic Aorta
C. Apostolidis, E. Johnson, H. Berhane, D. Dushfunian, S.
Cohn, B. Allen, A. Katsaggelos, M. Markl
Northwestern University, Chicago, United States
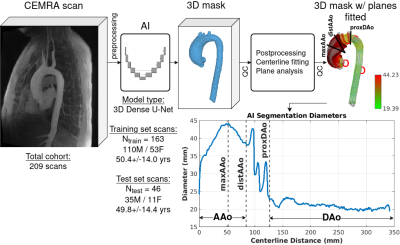
Impact: Aortic disease risk assessment relies on
imaging-based aortic diameter surveillance. We have
developed an automated, AI-driven diameter quantification
pipeline, aiming to improve manual processing speed and
reproducibility. We have achieved moderate agreement with
manual ground truth.
|
|
 |
Computer Number: 3
4271. Deep
learning-based segmentation of left ventricular myocardium in
cardiac magnetic resonance elastography
V. Atamaniuk, M. Anders, M. Obrzut, A. Pozaruk, Ł. Hańczyk,
B. Obrzut, M. Skoczylas, I. Sack, M. Cholewa
University of Rzeszow, Rzeszow, Poland
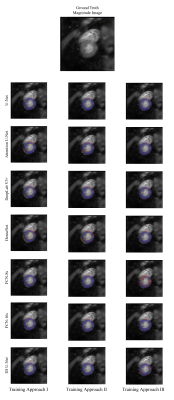
Impact: This study demonstrates deep learning’s
capability to automate left ventricular myocardium
segmentation in cardiac MR elastography, enabling faster and
more consistent myocardial stiffness assessments. Such
advancements could enhance cardiovascular disease
diagnostics, paving the way for improved clinical
decision-making in cardiology.
|
|
 |
Computer Number: 4
4272. Automated
carotid artery atherosclerotic inflammation segmentation on
PET-MRI: Mitigating partial volume effect
R. Li, A. Jha, P. Woodard, J. Zheng
Washington University in St. Louis, St. Louis, United States
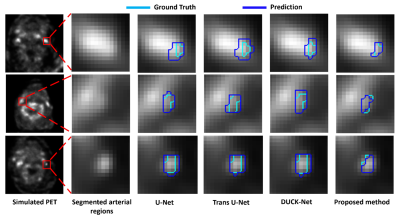
Impact: By improving the assessment of carotid
inflammation, this methodology has the potential to inform
clinical decisions and interventions, ultimately reducing
cardiovascular risk and mortality.
|
|
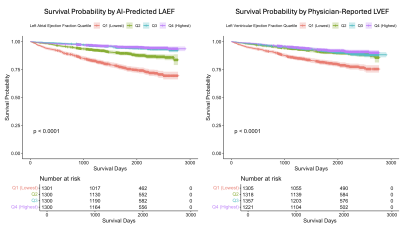 |
Computer Number: 5
4273. Automated
left atrial function analysis using AI is a stronger predictor
of survival than physician-measured left ventricular ejection
fraction
H. C. Cheung, S. Zaman, K. Vimalesvaran, K. Chow, P.
Kellman, H. Xue, R. Davies, G. D. Cole, C. Manisty, J. C.
Moon, J. P. Howard
Imperial College London, London, United Kingdom
Impact: Atrial function measured automatically using
inline AI is a strong and incremental predictor of patient
survival. This enables new biomarkers to be easily
translated into clinical workflow for improved patient care.
|
|
 |
Computer Number: 6
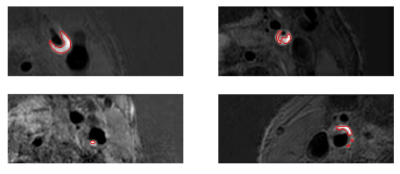
4274. Deep
Learning-Based Quantification of Intraplaque Hemorrhage Reveals
Impacts on Long-Term Carotid Plaque Progression
Y. Guo, D. Hippe, X. Wang, G. Canton, K. Zhang, A. Tang, M.
Ferguson, M. Mossa-Basha, N. Balu, T. Hatsukami, C. Yuan
University of Washington, Seattle, United States
Impact: This study underscores the significance of IPH
in carotid plaque progression, offering a precise deep
learning-based tool for monitoring. It enables more
effective risk assessment and personalized management
strategies, sparking new research into long-term IPH effects
on asymptomatic patients.
|
|
 |
Computer Number: 7
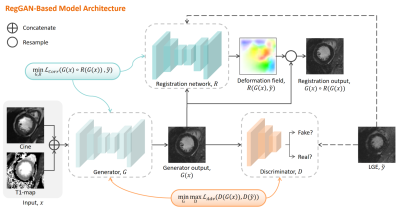
4275. Artificial
Intelligence for Gadolinium-Free CMR Tissue Charactization Using
Deep Learning–Based Virtual Native Enhancement
X. Jiang, Y. Wu, Y. Wang, T. Liu
The First Hospital of China Medical University, Shenyang, China
Impact: Virtual LGE has substantial potential to replace
LGE in diagnosing various cardiovascular diseases, providing
a more rapid, cost-effective scan and eliminating contrast
agent risks.
|
|
 |
Computer Number: 8
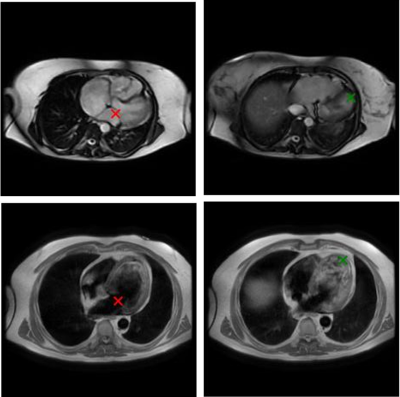
4276. Cardiac
landmark localization on axial stacks without dedicated ground
truth
G. Delso, E. Ali, J. Names, D. Rettmann, M. Janich
GE HealthCare, Barcelona, Spain
Impact: The proposed approach eliminates the need for
manual annotations for the training of some cardiac models.
Using outputs from existing long-axis models as surrogate
ground truth simplifies the creation and maintenance of the
training database.
|
|
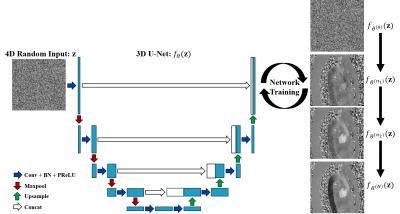 |
Computer Number: 9
4277. Unsupervised
phase unwrapping for aortic 4D flow MRI using deep image prior
Y. Ren, H. Hong, Z. Zhou, P. Hu
ShanghaiTech University, Shanghai, China
Impact: PUDIP outperforms the conventional methods and
can yield accurate flow velocity quantifications for 4D flow
MRI.
|
|
 |
Computer Number: 10
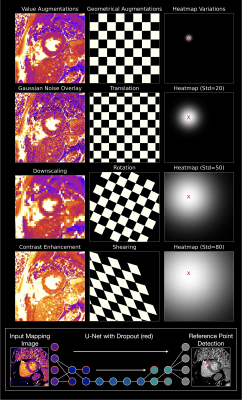
4278. Co-Evolution
of CNNs and Learning Environments for Automated Post-Processing
of T2 Parametric Mapping in Cardiovascular Magnetic Resonance
T. Hadler, C. Ammann, P. Reisdorf, S. Lange, J.
Schulz-Menger
Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany, Berlin, Germany
Impact: The evolutionary algorithm improves CNN
performance in cardiovascular MRI by dynamically optimizing
learning environments, enhancing adaptability across varied
imaging conditions. This approach strengthens model
generalizability and reliability, making it a promising
method for advancing robust AI tools in clinical practice.
|
|
 |
Computer Number: 11
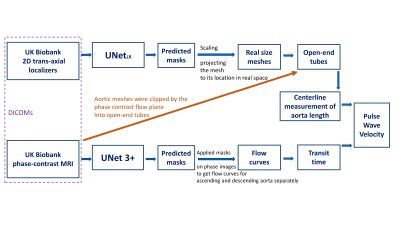
4279. Automated
Deep Learning Pipeline for Pulse Wave Velocity Measurement in UK
Biobank MRI Data
Y. Jiang, T. Yao, K. Punjabi, D. Knight, J. Steeden, R.
Davies, V. Muthurangu
University College London, London, United Kingdom
Impact: Our model enables automatic aortic arch PWV
measurement for UK Biobank subjects, which can be used in
the investigation of arterial stiffness and prediction of
cardiovascular disease for a large population.
|
|
 |
Computer Number: 12
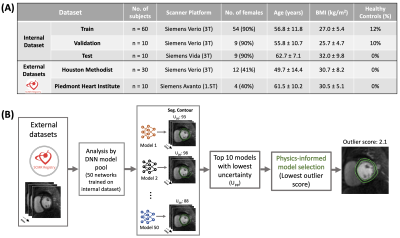
4280. Physics-informed
Model Selection for Robust Deep Learning Segmentation of
Multi-center Perfusion CMR: Initial Findings from the SCMR
Registry
D. M. Yalcinkaya, A. M. Sohi, K. Youssef, L. Zamudio, M.
Elliott, V. Polsani, R. Dharmakumar, R. Judd, M. Tong, D.
Shah, O. Simonetti, B. Sharif
Purdue University, West Lafayette, United States
Impact: The proposed hybrid approach has the potential
to improve the reliability of fully automated stress/rest
FPP analysis in clinical settings and in multi-center
clinical trials.
|
|
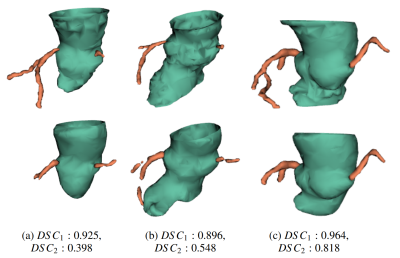 |
Computer Number: 13
4281. Contrast-Free
CMR-based Transcatheter Aortic Valve Implantation Planning:
Aortic Annulus and Coronary Ostias detection
E. Almar-Munoz, M. Pamminger, C. Kremser, M. Haltmeier, A.
Mayr
Medical University of Innsbruck, Innsbruck, Austria
Impact: Our CMR based TAVI planning algorithm advances
by automating pre-procedural assessments without iodinated
contrast. For the AA segmentation, the double-network
architecture and the unwrapping technique effectively
address the challenge of segmenting a virtual plane. Blind
tests reafirm our results.
|
|
 |
Computer Number: 14
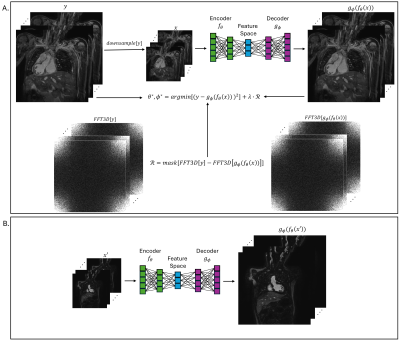
4282. Arbitrary
Factor Super-Resolution for 3D Whole-Heart MRI Using a
Frequency-Domain Informed Neural Network
C. Maciel, Q. Zou
University of Texas Southwestern Medical Center, Fort Worth, United States
Impact: By
implementing frequency-domain regularization inform network
training and arbitrary factor super-resolution, the proposed
method offers the potential to decrease acquisition time in
3D whole-heart MRI, while maintaining fine image detail
important for diagnostic utility.
|
|
 |
Computer Number: 15
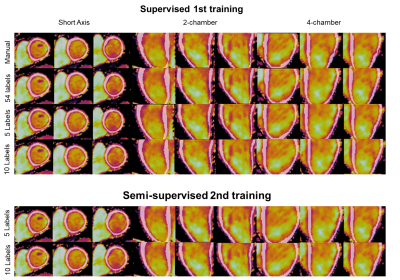
4283. Semi-supervised
3D Myocardial Segmentation for Whole-Heart Joint T1/T2 mapping
with Self-trained nnUNet
C. Rivera, A. Hua, R. Botnar, C. Prieto
IMPACT, Center of Interventional Medicine for Precision and Advanced Cellular Therapy, Santiago, Chile
Impact: Semi-supervised 3D nnUNet enables accurate
myocardial segmentation in 3D whole-heart joint T1/T2
mapping, even with limited labeled data. This could improve
efficiency, reduce manual segmentation effort, and
accelerate the diagnosis of myocardial diseases.
|
|
 |
Computer Number: 16
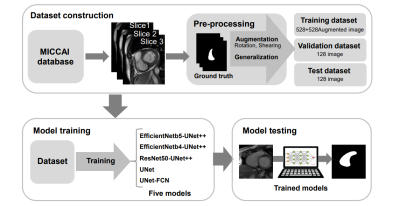
4284. Improve
the Accuracy of Right Ventricle Segmentation in Cardiac Magnetic
Resonance Images by UNet++
C-W Lin, H-H Peng
National Tsing Hua University, New Taipei City, Taiwan
Impact: The UNet++ model combined with special encoders
provided a robust solution to RV segmentation, with the
potential to enhance cardiac imaging analysis and support
clinical decision-making by reducing manual intervention and
increasing accuracy in heart function assessment.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.