Oral
Multimodal, Multi-Contrast fMRI
ISMRM & ISMRT Annual Meeting & Exhibition • 10-15 May 2025 • Honolulu, Hawai'i

| 15:45 |
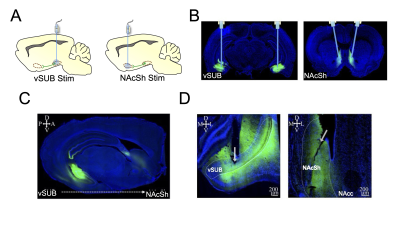 |
1005. Optogenetic
stimulation of cell bodies versus axonal terminals yields
remarkably comparable activity and functional connectivity in
the brain
L. Hsu, D. Cerri, Y-Y Shih
University of North Carolina at Chapel Hill, Chapel Hill, United States
Impact: The fMRI findings from this study challenge the
general assumption that optogenetic stimulation at synaptic
terminals primarily recruits feedforward activity in
downstream brain regions, urging the neuromodulation
community to exercise caution in interpreting data obtained
with terminal optogenetic approach.
|
| 15:57 |
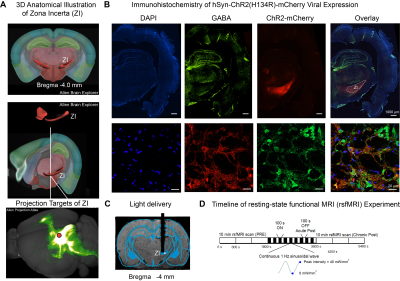 |
1006. Optogenetic
fMRI Investigation of the Role of Zona Incerta in Regulating
Brain-wide Functional Connectivity

J. Wen, L. Xie, X. Wang, T. Ma, P. Cao, E. X. Wu, A. T.
L. Leong
The University of Hong Kong, Hong Kong SAR, China
Impact: We posit that the findings from this study will
provide critical insights into where and how ZI neural
activities are distributed throughout the brain, and their
contributions to brain functional connectivity.
|
| 16:09 |
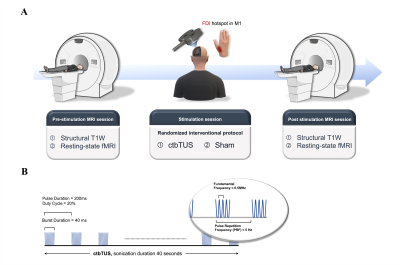 |
1007. Synthesized
skull properties relate to functional changes at rest due to
low-intensity focused ultrasound stimulation on M1
K-H Chen, W-C Yang, H-L Liu, Y-C Kuo, Y-S Dong, Y-C Shih
National Taiwan University Hospital Hsin-Chu Branch, Hsin-Chu City, Taiwan
Impact: Shedding light
on the feasibility of T1-based-pseudo-CT skull properties to
predict neuromodulatory effect of ctbTUS.
|
| 16:21 |
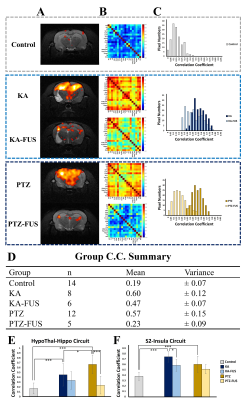 |
1008. Exploring
Epileptic Connectivity: A Comparative Study of Kainic Acid and
Pentylenetetrazol Using rs-fMRI and Focused Ultrasound
P-C Chu, W-H Ruan, H-L Liu, J-H Chen
Graduate Institute of Biomedical Electronics and Bioinformatics, National Taiwan University, Taipei, Taiwan
Impact: This study positions resting-state fMRI as a
valuable biomarker for epilepsy. Combined with FUS, which
modulates neural circuits, it enhances our understanding of
epilepsy mechanisms and brain signaling pathways, offering
insights for more targeted treatments.
|
| 16:33 |
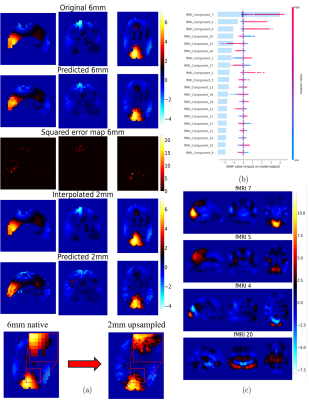 |
1009. Enhancing
brain activity mapping through BOLD-fMRI and MEG data fusion
using explainable machine learning.

J. Benacek, K. Singh, D. Jones, D. Marshall, S. Rushton,
M. Palombo
Cardiff University, Cardiff, United Kingdom
Impact: Our proof-of-concept approach demonstrates a
strong predictive relationship between fMRI and MEG data.
Using explainable machine learning, we generated
high-resolution MEG maps from fMRI inputs, and provided
data-driven insights into their relationship, opening new
avenues for investigating neurovascular coupling.
|
| 16:45 |
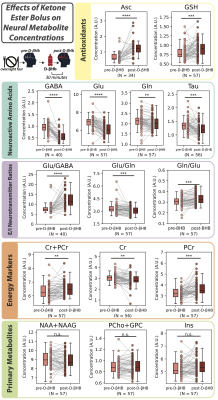 |
1010. Ketosis
Elevates Antioxidants and Enhances Neural Function Through
Improved Bioenergetics: A 1H MR Spectroscopy Study

H. van Nieuwenhuizen, B. Antal, A. Hone-Blanchet, A.
Lithen, L. McMahon, S. Nikolaidou, Z. Kuang, K. Clarke,
B. Jenkins, D. Rothman, L. Mujica-Parodi, E-M Ratai
Stony Brook University, Stony Brook, United States
Impact: The combination of metabolic and functional
neuroimaging data in our study provides a comprehensive view
of how ketosis affects brain chemistry and functional
network dynamics, offering insights for developing novel
treatment strategies for a variety of psychiatric and
neurodegenerative disorders.
|
| 16:57 |
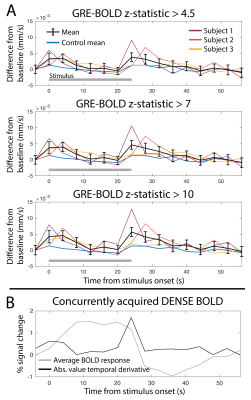 |
1011. Quantification
of cerebral cortical displacement driven by visual stimulation
using motion-encoded stimulated-echo EPI at 7T

A. Strom, T. Reese, L. Lewis, J. Polimeni
Massachusetts Institute of Technology, Cambridge, United States
Impact: Cerebral cortical tissue motion in response to
neuronal activation is quantified in
vivo in real-time, enabling investigations into the
contributions of tissue displacement to CSF flow patterns
and characterization of dynamic partial volume effects for
fMRI analysis and interpretation.
|
| 17:09 |
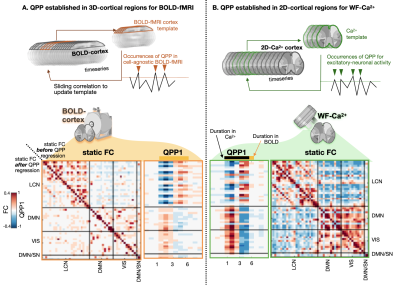 |
1012. Neural
activity underpins dynamic BOLD signals: findings from
simultaneous wide-field fluorescent calcium imaging and fMRI.
F. Mandino, C. Horien, X. Shen, X. Papademetris, S.
Keilholz, N. Xu, E. Lake
Yale School of Medicine, New Haven, United States
Impact: Quasi-periodic patterns (QPPs), measured via
BOLD-fMRI, are recurring low-frequency coordinated waves of
brain activity that appear in humans and model species.
Here, we extend the characterization of the neural
underpinnings of QPPs using simultaneous wide-field
fluorescent calcium imaging and BOLD-fMRI.
|
| 17:21 |
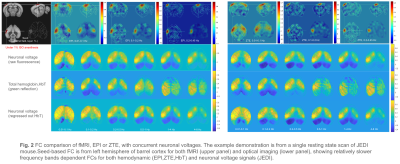 |
1013. Seamless
integration of fMRI (EPI, ZTE) and wild-field optical imaging in
mice
W-J Pan, L. Daley, L. Meyer-Baese, D. Jaeger, K. Gopinath,
S. Keilholz
Emory University/Georgia Institute of Technology, Atlanta, United States
Impact: Our results demonstrate the feasibility of the
seamless integration of fMRI and optical imaging to the era
of multi-modality neuroimaging.
|
| 17:33 |
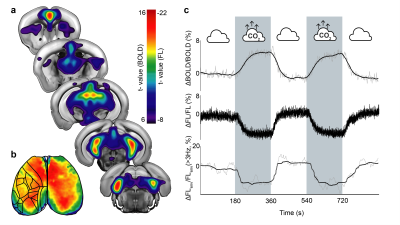 |
1014. Simultaneous
fMRI and fluorescence calcium imaging of brain dynamics under
hypercapnia in mice
I. Gezginer, Z. Chen, Y. Chen, D. Razansky
ETH Zurich, Zurich , Switzerland
Impact: By differentiating neural and vascular responses
under hypercapnia, this study highlights limitations of BOLD
as a neural marker during CO₂ stress. Findings inform
strategies to restore neurovascular balance, relevant to
chronic hypercapnic disorder diagnosis and treatment.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.