Oral
The Brain Through Development
ISMRM & ISMRT Annual Meeting & Exhibition • 10-15 May 2025 • Honolulu, Hawai'i

| 15:45 |
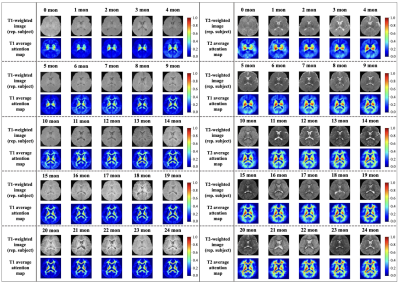 |
1035. Attention-Guided
Deep Learning model focusing on Myelination for Predicting
Pediatric Brain Age using Multi contrast MRI
C. Ryu, S. Jung, N-Y Shin, D-H Kim
Yonsei University, Seoul, Korea, Republic of
Impact: This model improves pediatric brain age
prediction by accurately identifying myelination regions,
providing clinicians with enhanced insights into early
neurodevelopmental progress. This approach presents
potential for early intervention and monitoring of
neurodevelopmental disorders, developing clinical resources
in pediatric neurology.
|
| 15:57 |
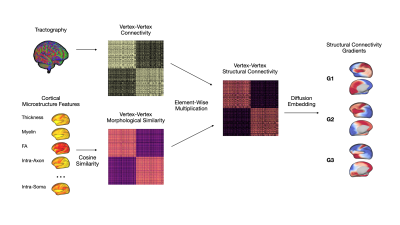 |
1036. Structural
Gradients of the Developing Brain Connectome
J. Lee, K. M. Huynh, H. Taylor, J. Mao, G. Lin, S. Ahmad,
P-T Yap
University of North Carolina at Chapel Hill, Chapel Hill, United States
Impact: For the first time at the vertex level, we map
the development of neocortical structural gradients during
the first five years of life, enabling fine-scale study of
the functional-structural connectome relationship.
|
| 16:09 |
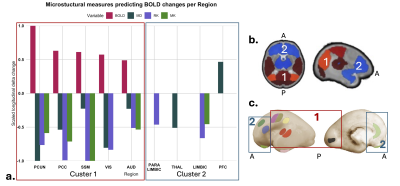 |
1037. Increased
BOLD variability is accompanied by changes in tissue
microstructure and upregulation of gliogenesis in the preterm
infant cortex

J. Sa de Almeida, A. Boehringer, S. Loukas, E. Fischi,
A. Van Der Veek, L. Lordier, S. Courvoisier, F.
Lazeyras, D. Van De Ville, G. Ball, P. Hüppi
University Hospitals of Geneva, Geneva, Switzerland
Impact: Preterm birth disrupts cortical BOLD variability
and microstructure by term-equivalent-age. During preterm
infants’ neurodevelopment, an increase in cortical BOLD
variability reflects ongoing changes to tissue
microstructure and an upregulation of genes mediating
gliogenesis, identifying putative mechanisms for preterm
brain injury.
|
| 16:21 |
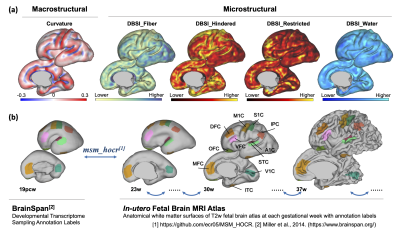 |
1038. Unveiling
Fetal Cortical Folding: Neuroimaging and Genetic Insights

X. Xu, R. Chen, T. Zheng, Z. Zhao, M. Li, D. Wu
College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China
Impact:
This research links specific genes to fetal cortical folding, offering insights into prenatal brain development and potential biomarkers for early neurodevelopmental disorder detection. These findings enable further study of gene-environment interactions, advancing diagnostic and therapeutic approaches. |
| 16:33 |
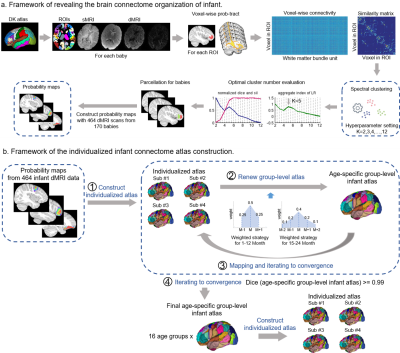 |
1039. Precision
connectome mapping in infants
M. Han, T. Zhao, Y. He
Beijing Normal University, Beijing, China
Impact:
We provide a refined atlas of cortical and subcortical segmentations at both the individual and finely aged group levels for infants and toddlers, offering an essential foundational and common tool for pediatric brain image analysis and brain development research. |
| 16:45 |
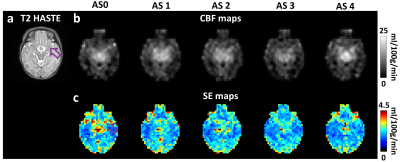 |
1040. ASL
perfusion MRI in neonates less than one week of age: arterial
suppression and vector-projection-based CBF estimation
Z. Hu, J. Shepard, M. Guryildirim, Y. Uchida, K. Oishi, W.
Shi, P. Liu, V. Yedavalli, A. Tekes, D. Lin, W. C. Golden,
H. Lu
Johns Hopkins University School of Medicine, Baltimore, United States
Impact: Using the proposed ASL acquisition and
processing method, high-fidelity perfusion maps can be
obtained from early neonates in less than 4 minutes. This
technique holds potentials for studying neonatal brain
diseases involving perfusion abnormalities.
|
| 16:57 |
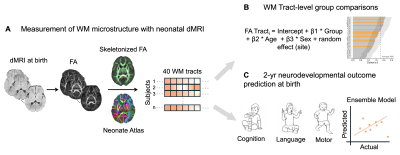 |
1041. Accurate
prediction of 2-year-old neurodevelopmental outcomes in mild HIE
with neonatal white matter microstructure.
S. Mohapatra, W. Wu, K. Sindabizera, L. Chalak, H. Huang, M.
Ouyang
Children's Hospital of Philadelphia, Philadelphia, United States
Impact: This study establishes that diffusion metrics
can identify white matter tract alterations in mild HIE at
birth, and ML offers reliable prediction of future
neurodevelopmental outcomes expected at age 2. Early
identification enables tailored interventions, benefiting
patient outcomes significantly.
|
| 17:09 |
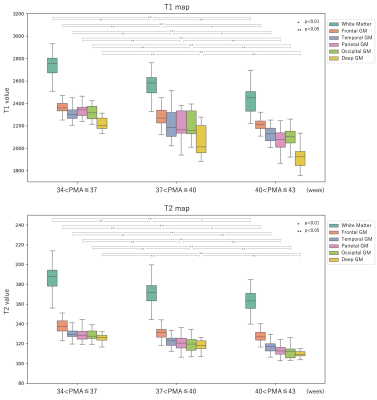 |
1042. Mapping
Brain Maturation in Neonates: A Quantitative Study with MR
Fingerprinting
A. Kato, N. Aida, J. Shibasaki, K. Murata, M. Nittka, G.
Koerzdoerfer, K. Nozawa, D. Utsunomiya
Yokohama City University, Yokohama, Japan
Impact: This study demonstrates that MRF-derived
quantitative values vary regionally at different rates based
on postmenstrual age in neonates. MRF allows precise
tracking of these developmental changes, underscoring its
potential for assessing brain maturation during early
development.
|
| 17:21 |
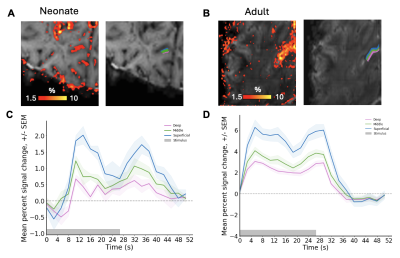 |
1043. Ultra-high
field fMRI characterisation of cortical depth dependent BOLD
responses in the primary visual cortex in neonates
A. Massmann, E. Pickles, P. Bridgen, P. Di Cio, L.
Billimoria, I. Tomazinho, C. Da Costa, D. Gallo, A. Edwards,
J. Hajnal, S. Malik, T. Arichi, J. Willers Moore
King's College London, London, United Kingdom
Impact: We demonstrated the first robust positive BOLD
response at 7T in the neonatal visual system comparable to
adults. Characterising the differences in depth-dependent
activation gives us an insight into the developing
neurobiology of the visual system.
|
| 17:33 |
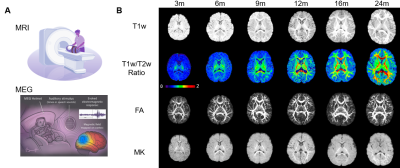 |
1044. Unveiling
differential maturation of infant visual and auditory cortex
through multimodal MRI and MEG

R. Li, T. Zhu, Z. Zhang, K. Sindabizera, Y. Chen, J. C.
Edgar, M. Ouyang, H. Huang
Children's Hospital of Philadelphia, Philadelphia, United States
Impact: Revealing the differential structural and
functional maturation trajectories of the auditory and
visual systems using multimodal MRI and task-based MEG in a
large infant cohort may provide insights into both typical
brain development and brain disorders.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.