Ian Robert Young OBE PhD DSc (Hon) FRS FREng FIEE FlnstP FRCR (Hon) FRCP (Hon)
11 January 1932 – 27 September 2019
Ian Young (Fig. 1) was born in London on 11 January 1932, the eldest of three sons. His father, John Stirling Young, was Regius Professor of Pathology at the University of Aberdeen from 1937 to 1962. Ian was educated at Sedbergh School in Cumbria, North West England and studied natural philosophy at the University of Aberdeen. He completed his BSc in 1954, and his PhD in Optical Metrology at the University under the supervision of Reginald V Jones FRS (1911-1997), who was famous for many feats of scientific intelligence and the development of electronic countermeasures during World War II.
EMI and Computed Tomography (CT)
After working for Hilger and Watts Ltd, Evershed and Vignoles Ltd, and Evershed Power Optics Ltd, Ian joined the Electric and Musical Industries (EMI) Central Research Laboratory (CRL) in August 1976. EMI was established in 1931 as a vertically integrated company producing sound recordings as well as recording and playback equipment. To this was added TV broadcasting and stereo sound, as well as radar during World War II under the direction of Alan Blumlein. After the war the company specialised in entertainment and defence until quite unexpectedly, and at very little cost to the company, Sir Godfrey Hounsfield FRS developed the world's first head CT scanner and began clinical imaging with it at Atkinson Morley's Hospital, London in October 1971. This transformed neuroradiology. In 1975 he followed this with the CT5000 whole body scanner which had wide application in oncology as well as chest and musculoskeletal disease. CT was a major departure for the company which had no significant track record in X-ray technology or medical equipment. Following the invention of MRI by Paul Lauterbur in 1973, EMI decided to expand its role in medical imaging and established a Nuclear Magnetic Resonance (NMR) group.
0.1T Walker MRI System at the EMI CRL
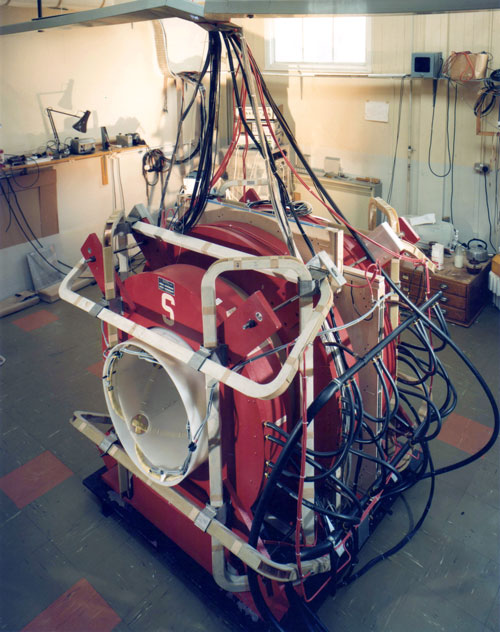
Fig. 2 (1979).EMI CRL MRI system based on a Walker 0.1T resistive phantom magnet with the South Pole (S) shown and shim coils outside the magnet. Animal and human volunteer studies were performed with this system.
The NMR group began with a Walker 0.1T resistive magnet (Fig. 2) and, under the direction of Hugh Clow and Ian Young, produced the world's first published human MR image of the brain in November 1978. This was followed by an inversion recovery (IR) image of Ian Young's brain in Autumn 1979 which showed very high grey-white matter contrast. The image was shown by Godfrey Hounsfield during his Nobel prize lecture on Computed Medical Imaging on 8 December 1979, together with Godfrey's explanation of its contrast in terms of differences in tissue and fluid T1s (Figs. 3A and 3B) (1).
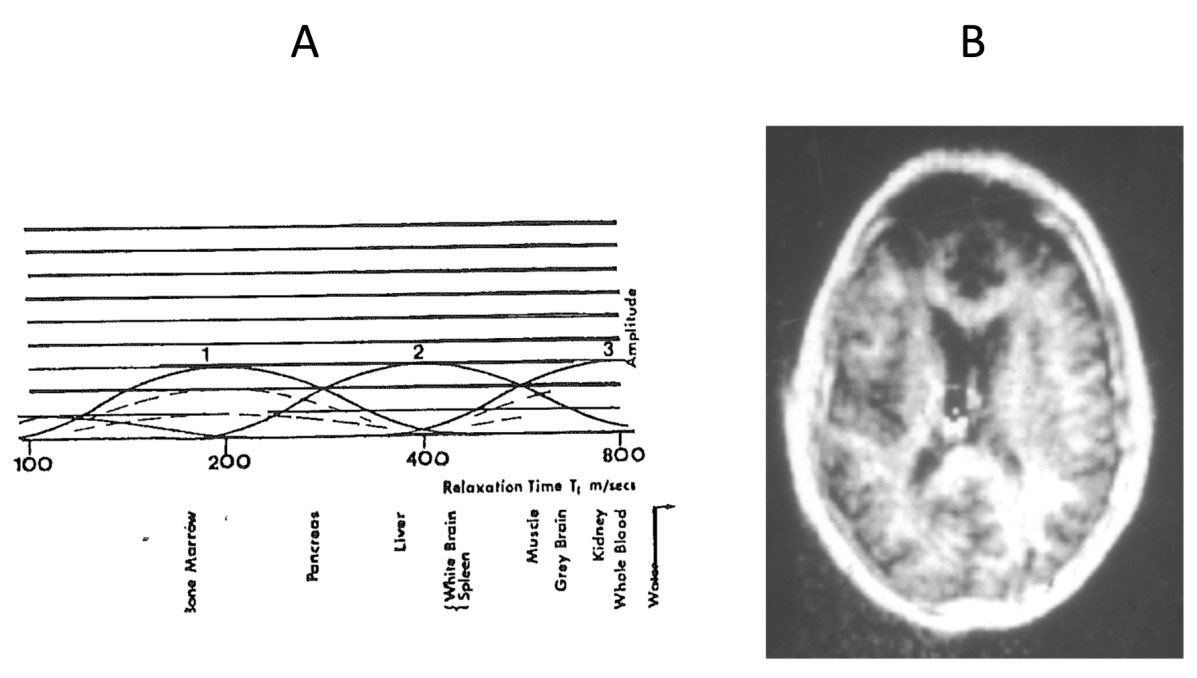
Fig. 3 (1979). Godfrey Hounsfield's diagram relating signal amplitude to the log of tissue T1 including White Brain and Grey Brain (A). Godfrey presented this during his Nobel Prize Lecture on 8 December, 1979. The curve opposite the numeral 2 shows White Brain with higher amplitude than Grey Brain which is what is seen on the IR image (TR/TI = 1000/300 ms) of Ian Young's brain ("Ian") (B) obtained at the Central Research Laboratory (CRL) of EMI Ltd in Autumn 1979.
In 1980 Ian Young added to the IR sequence, gradient echo and spin echo (SE) sequences which produced images of similar quality, and studied volunteers with Jackie Pennock, John Gore and Frank Doyle.
The Cryogenic MRI System, Neptune
With funding from the Scientific and Technical Services Branch of the Department of Health and Social Security headed by Gordon Higson (2), Ian's group built a 0.15T system based on the world's first commercial large bore cryomagnet (Fig. 4). The magnet was constructed by Oxford Instruments headed by Sir Martin Wood FRS (3). The MR system was shifted to Hammersmith Hospital, London in January 1981, and patient imaging began there in March 1981. The cryogenic system looked very different from the Walker system (Fig. 2) and had the appearance of a modern MR system.
Ian established a successful clinico-industrial group with Picker International at Hammersmith hospital which coordinated clinical work in the hospital with technique development in what became the dominant mode for clinical research in MRI thereafter.
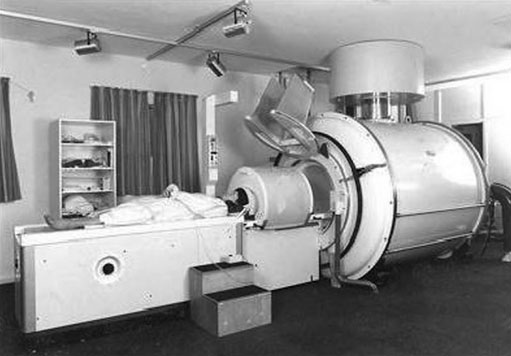
Fig. 4 (1981). Cryogenic system based on a 0.15T magnet built by Oxford Instruments and installed at Hammersmith Hospital in January 1981. This is a dramatic technological advance from the Walker resistive system (Fig. 2); it has the appearance of a modern clinical MRI system.
Comparison with CT
It was very clear at this time that if MRI was to attract the large scale investment necessary to develop it into a standard clinical product, the technique would have to show something of clinical importance that CT could not do, i.e. a "decisive clinical advantage" or what later became known as a "Killer App".
This was not an easy task. Brian Worthington FRS from Nottingham said in 1996 "CT had developed rapidly in the period from 1974 to the early 1980s and this was being compared with MRI, an embryonic technique. As a neuroradiologist I was appalled to see that the commonest primary benign tumour in the intracranial compartment, the meningioma, was invisible on MRI. I had struggled to try and separate tumour from oedema which was easily done with contrast in CT, but we were having great difficulty with MRI. By mid 1982 there were some very difficult problems to be addressed" (4).
Ian Isherwood neuroradiologist from Manchester shared his views: "By the time we get to 1981 or 1982 many people felt that CT had reached a plateau of excellent spatial resolution. The images were excellent. They were understandable not only by radiologists but by the clinicians who requested the examinations. The MR images which were then available were very poor by comparison" (4).
In spite of these problems, in November 1981 Ian published a study of 10 Multiple Sclerosis (MS) patients in which 112 lesions were detected with MRI using an IR sequence and only 19 were seen with CT (5). It was a quantifiable, decisive clinical advantage for MRI over CT, and led to large scale commercial investment in research and development of the technique. Oxford Instruments' order book for magnets increased from £1M in 1981 to £25M in 1982 (6).
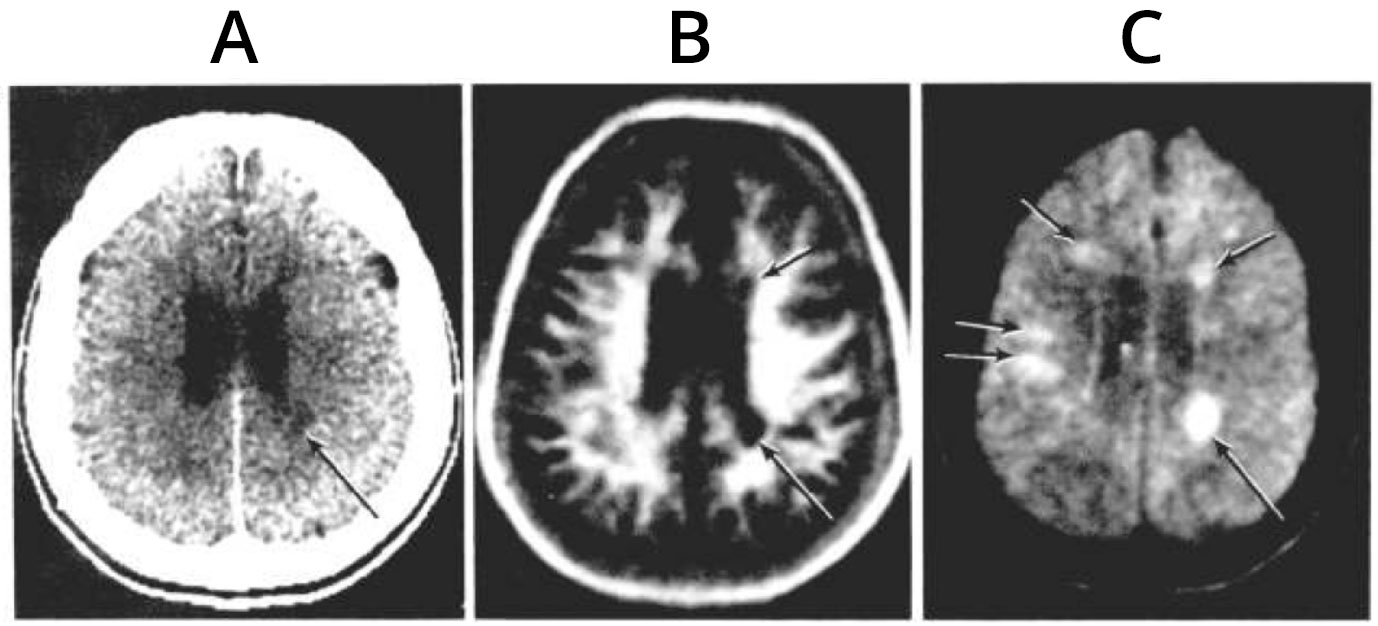
Fig. 5 (1982). Multiple Sclerosis. CT (A), IR (TR/TI = 1400/400 ms) (B) and SE (TR/TE = 1000/80 ms) (C) images. A single lesion is seen on the CT scan (long arrow) (A). This lesion (long arrow) and at least three other lesions are seen on the IR image (e.g. short arrow) (B), and at least five lesions are seen on the SE image (long and short arrows) (C).
The T2-weighted SE Sequence
The success with the IR sequence was followed by David Bailes' development of the heavily T2-weighted SE sequence. This was used in a study of 32 brain patients that was published in July 1982 (Fig. 5) (7) as well as for studies of the posterior fossa where CT was degraded by beam hardening artefacts and MRI had a significant clinical advantage, providing that meningiomas were excluded with contrast enhanced CT.
The Multi Sequence Approach
The use of the Spin-warp data acquisition developed by Jim Hutchison and Bill Edelstein from Aberdeen (8) allowed imaging in the sagittal plane and provided iconic images of the brain with white matter appearing white, grey matter appearing grey and CSF appearing black, corresponding to the post-mortem appearance of the brain (Fig. 6). With CT, the sagittal plane was not directly attainable, the grey-white matter contrast was reversed i.e. grey matter appeared white, and white matter appeared grey, and the contrast between them was lower.
The Multi Sequence Approach using T1-weighted, T2-weighted, and other sequences to show anatomy in the first instance, and highlight disease in the second, was used thereafter in studies of the brain (9,10). Previously only a single sequence e.g. Steady State Free Precession (SSFP) had been used for clinical studies of the brain (e.g. 11).
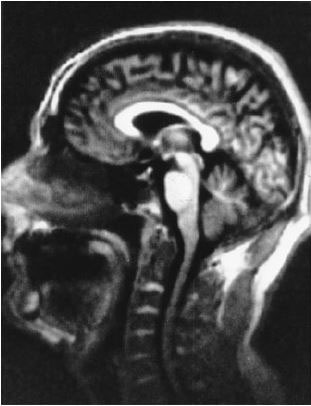
Fig. 6 (1982). Sagittal IR image of the brain using the Spin-warp data acquisition (TR/TI = 1400/400 ms). This is a classic anatomical view with white matter appearing white, grey matter appearing grey and CSF appearing black. It was not attainable with projection reconstruction due to B0 field inhomogeneity in the Z direction. Images of this type became iconic.
The "Field Wars"
However, the MR imaging world was turned on its head at the November 1982 meeting of the RSNA when General Electric (GE) showed high quality brain images obtained at 1.5T, ten times the field strength of 0.15T that Ian and others were operating at. The GE 1.5T system was aggressively marketed even though the company had no product to sell. Customers were strongly advised not to buy other companies' lower field systems, but instead to buy the 1.5T GE product as soon as it became available.
This was the beginning of the "field wars" which dominated MRI for the next 4-5 years. Ian and his group persisted at 0.15T, developed about 30 new receiver coils with the help of Walter Curati (e.g. Fig. 7), used low bandwidth acquisitions, performed phase sensitive flow imaging with David Firmin and Donald Longmore (12), implemented the short inversion time (STIR) pulse sequence which suppressed fat signals and produced very high contrast images of abnormalities (13), and developed motion artefact control techniques (e.g. Respiratory Ordered Phase Encoding, ROPE) (14).

Fig. 7 (1983). A spherical "Jedi" coil. These were made in about ten different sizes to allow close fitting for neonates, children and adults. They provided improved signal to noise ratios compared with the original single adult sized saddle coil.
There were problems at high field; images were degraded much more by artefacts from susceptibility, chemical shift and motion than at low field. Over time Ian, Jackie Pennock, Di Spencer, Judith Payne and others showed that the field argument was not a cut and dried matter; it was possible to perform clinically competitive studies at low field using much cheaper MRI systems than 1.5T ones.
Contrast Agents
Ian's group also used the contrast agent Gadolinium-DTPA developed by Hanns-Joachim Weinmann of Schering (15) and performed the first clinical study with this agent (16) as well as subsequent studies of meningiomas which were well demonstrated (17), and many other diseases. This allowed comprehensive stand-alone MRI examinations of the brain rather than just studies for MS or posterior fossa disease after meningiomas had been excluded with contrast enhanced CT. Gadolinium-DTPA was of particular value in the study of CNS tumours that did not display an increase in T1 or T2 (18). The agent also opened up other applications of MRI in body oncology including breast disease.
Susceptibility
Perhaps the most curious of all the developments during this time was susceptibility weighted imaging (SWI). When GE eventually produced 1.5T clinical systems one of the first applications was intracranial haematomas. Bob Grossman and John Gomori of the University of Pennsylvania identified central and peripheral low signal areas at different stages of haemorrhage and attributed them to susceptibility effects. They argued that these would not be visible at low fields because susceptibility effects increased with the square of the field and therefore would be, for example, 100 times less obvious at 0.15T (19). This was therefore thought to be a "Killer App" for high field. In fact, the low signal areas could be well seen at 0.15T (Fig. 8 ) using heavily T2-weighted gradient echo sequences and phase maps (20,21) which were much more sensitive to susceptibility than the T1 and T2-weighted SE sequences used by Grossman and Gomori in their study.
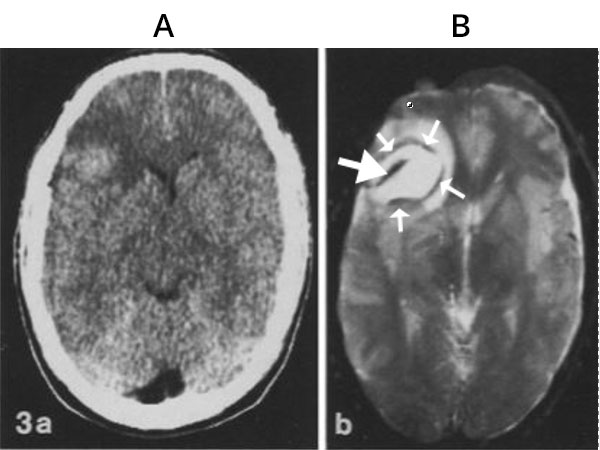
Fig. 8 (1986). Intracerebral haemorrhage. CT (A) and heavily T2-weighted gradient echo (TR/TE = 500/120 ms) image (B) obtained at 0.15T. The gradient echo image shows a central linear low signal area (thick arrow) as well as a low signal rim (thin arrows). These features of haemorrhage were not thought to be detectable at the low field of 0.15T.
SWI should have been an obvious high field application with major advantages at 1.5T and above, but instead it emerged as a low field discovery. Ian and his group published the first paper on mapping phase changes due to susceptibility (21) as documented by Jeff Duyn (22). This was performed at 0.15T. Ian's group wrote two further papers on the topic over the next two years before any other group published on the subject.
MRS (32)
David Bryant, Alan Collins, Reg Harman, Glyn Coutts and others in Ian's group built a 1.5T spectroscopy system. In conjunction with Jane Cox, Simon Taylor-Robinson, David Menon, Carol Peden, Jimmy Bell and Lousie Thomas as well as David Gadian and Richard Iles they published over 200 papers on proton MRS of the brain, liver 31P, neonatal brain, cardiac by-pass studies and anaesthesia.
Endocavity and Interventional MRI (33)
These approaches were developed by Nandita deSouza, Andy Williams and David Gilderdale of Ian's group who made internal anal, rectal, cervical, vaginal and prostate coils including paediatric adaptations. They showed a level of anatomical resolution and sensitivity to disease that was not achievable with external coils. This was extended to endoscopy and image guided biopsy. Nandita also developed thermal ablation in different forms for disease of the prostate and continued her work with David at the Institute of Cancer Research, London.
FLAIR, later T2-FLAIR
The Fluid Attenuated IR (FLAIR) sequence was developed by Jo Hajnal of Ian's group who used an inversion pulse to null CSF and doubled the TE of T2-weighted SE sequences to provide unambiguous high contrast visualisation of abnormalities in a wide range of disease of the brain (23) including MS (Fig. 9).
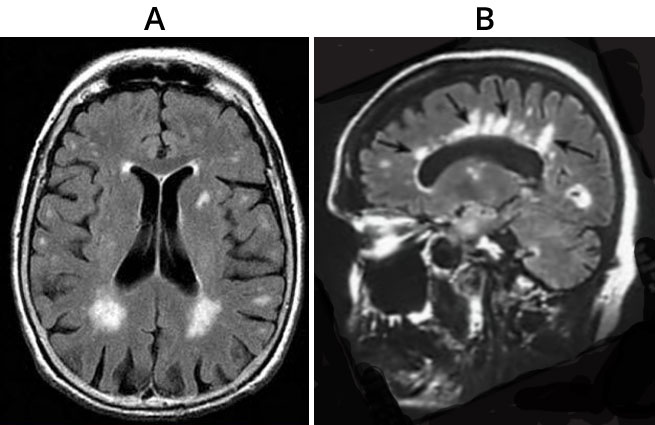
Fig. 9. Multiple sclerosis. T2-FLAIR (TR/TI/TE = 4000/2200/160ms) images in the transverse (A) and parasagittal (B) planes. Plaques (some shown with black arrows in [B]) are seen with high signal on both images.
Henry Marsh, a neurosurgeon who worked at Atkinson Morley's Hospital (the site of installation of Godfrey Hounsfield's first head CT scanner) and St George's Hospital wrote two books on his practice of neurosurgery which became best sellers. In his books "Do No Harm" and "Admissions" he wrote that CT and MRI were essential to his practice but the only technical term he mentioned about either technique was "FLAIR". It came up when he was instructing a new trainee who was floundering on what type of MR images she should look at to reach a correct diagnosis (24). The sequence attained very wide acceptance.
The Neonatal System
After publishing the first clinical MRI study in children in 1982 (25) and supporting a very active paediatric group centred on Lilly Dubowitz, Frances Cowan and Mary Rutherford for over a decade, Alasdair Hall used a very short bore (35cm), self-shielded 1.0T magnet built by Oxford Magnet Technology (OMT) to construct a dedicated neonatal MR system. It was installed in the Neonatal Intensive Therapy Unit (NITU) at Hammersmith Hospital in 1995 and allowed examination of very premature and very sick infants without requiring transport out of the NITU (26). These patients had previously not been examinable with MRI because of the risks in taking them elsewhere and providing the necessary monitoring and intensive care. Infants down to 24-25 weeks GA were examined, often repeatedly, and details of normal development and disease were established (27) with the help of Serena Counsell and later Christina Malamateniou. This work has been continued by David Edwards at Kings College, London.
The Department of Electrical and Electronic Engineering, Imperial College
After Ian retired from Hammersmith Hospital in 1997, he worked with Richard Syms and other members of the Electrical and Electronic Engineering department of Imperial College. His research focussed on novel magnet designs for mobile scanners, a magic angle scanning system designed by John McGinley and operated by Karyn Chappell and Mihailo Ristic (28,29), robotic tools for interventional MRI (particularly endoscopic retrograde cholangio-pancreatography and prostate biopsy), MR thermometry with Jeff Hands, internal imaging systems and the application of metamaterials to MRI.
The MR Societies: the Society for Magnetic Resonance in Medicine (SMRM), the International SMRM (ISMRM) and the Society for MRI (SMRI)
Ian was chairman of the local committee for the fourth SMRM meeting at the Barbican in 1985. The meeting is still remembered for Raymond Damadian's contribution. Ian was president of the SMRM from 1991-92 and secretary of the ISMRM from 1993-96. He was also secretary of the merger committee chaired by Herbert Kressel that integrated SMRM and SMRI and later helped organise the Glasgow ISMRM meeting in 2001. Ian received gold medals from the SMRM and SMRI as well as a silver medal from the ISMRM, and is the only person to have been honoured in this way.
The History of the Development of MRI and MRS in the UK (MRIS History UK, https://mrishistory.org.uk)
Ian helped establish an eBook to include historical accounts by authors from different disciplines and institutions in the UK. The contributions would be proof-read, critically edited and peer-reviewed. Ian wrote the first articles for the eBook which went through multiple revisions before being approved by the reviewers (34,35).
The eBook includes an exceptional contribution on localisation in MRI and MRS by Paul Bottomley from Nottingham (36) and a complementary account of work at Oxford by David Hoult (37). There are also a range of clinical accounts including St Mary's Hospital (Wady Gedroyc) (38) and Edinburgh (Joanne Wardlaw) (39) as well as MR history in general (Peter Rinck [40], Adrian Thomas [41]), the MRI societies (John Griffiths [42]), life working with Ian (43,44), experience as a trainee (45) and an obituary of Ian Young (46). The eBook is being continued by Martyn Paley (47) and Graeme Bydder (48).
Ian's Final Work
He concentrated on two things. The first was dealing with "weighting". This is the concept used clinically to relate the contrast seen on images to the difference or change in tissue property such as T1 and T2 thought to be responsible for the contrast. About three years after its introduction in 1983, the concept was described by the American College of Radiology (ACR) as "ambiguous" and "confusing" (30), and the ACR refused to list the terms T1-weighting and T2-weighting in their Glossary of Terms. However, the ACR did not propose a replacement, or refinement, of the concept although it is central to clinical image interpretation. Its use continues every day in clinical MRI in spite of the many contradictions and inconsistencies associated with it.
Following Godfrey Hounsfield's lead from 1979 (Fig. 3A) Ian used the Bloch equations to treat pulse sequences as tissue property filters (Fig. 10) and derive mathematical expressions for sequence and image weighting including the relative contribution of each tissue property to image contrast. The slope of the curve in Fig. 10 is the sequence weighting for the T1 segment of the SE sequence. Once the concepts are understood, the approach only requires a high school knowledge of calculus and the performance of electrical RLC filters but it resolves many of the problems with the conventional use of weighting and provides insights and research directions that are not apparent otherwise.
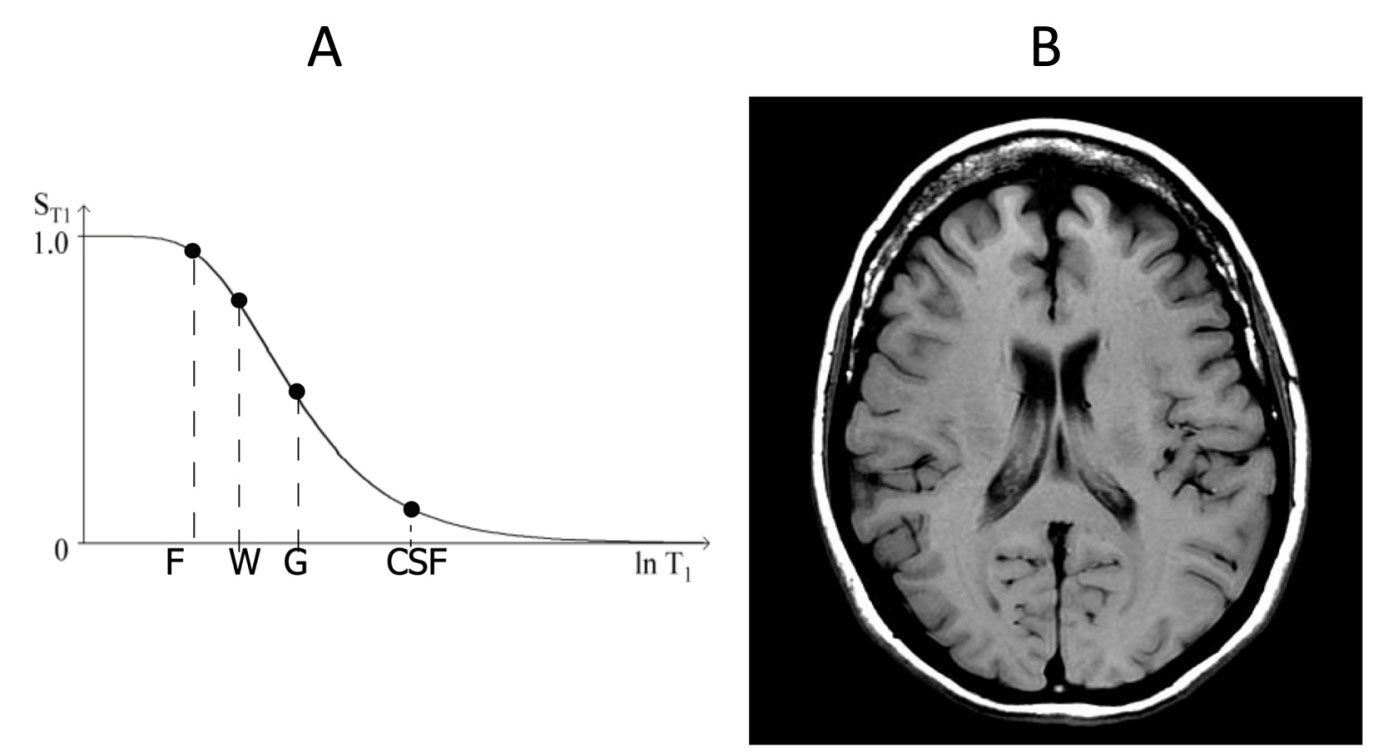
Fig. 10. Plot of signal vs T1 for the T1 filter of a SE sequence (A). The filter is low pass (i.e. low values of T1 are high signal and pass, and high values of T1 are low signal and are "stopped". The signals from fat (F), white matter (W) and grey matter (G) correspond to the brightness seen on the T1-weighted SE image (TR/TE = 500/8 ms) shown in (B).
His second initiative was to extend the IR sequence beyond the double IR (DIR) sequence described in 1985 and 1994 (13, 31) to combine two or more IR images in Multiplied, Added, Subtracted and/or fiTted (MASTIR) sequences. These provide new options for suppressing unwanted signals, and can nearly double again the already double contrast of the standard IR sequence over a particular range of T1 values by subtracting a magnitude reconstructed longer TI IR image from a shorter TI one as shown in Fig. 11 using the tissue property filters approach. The slope of the subtracted blue curve in the transition band of the filter is nearly twice that of the two contributing IR curves. A further IR pulse multiplication to suppress fluid can be added to give the Multiplied Subtracted IR (MSIR) sequence. It shows far more extensive abnormality in the central white matter of the brain in a case of MS than the conventional heavily T2-weighted SE sequence (Fig. 12).
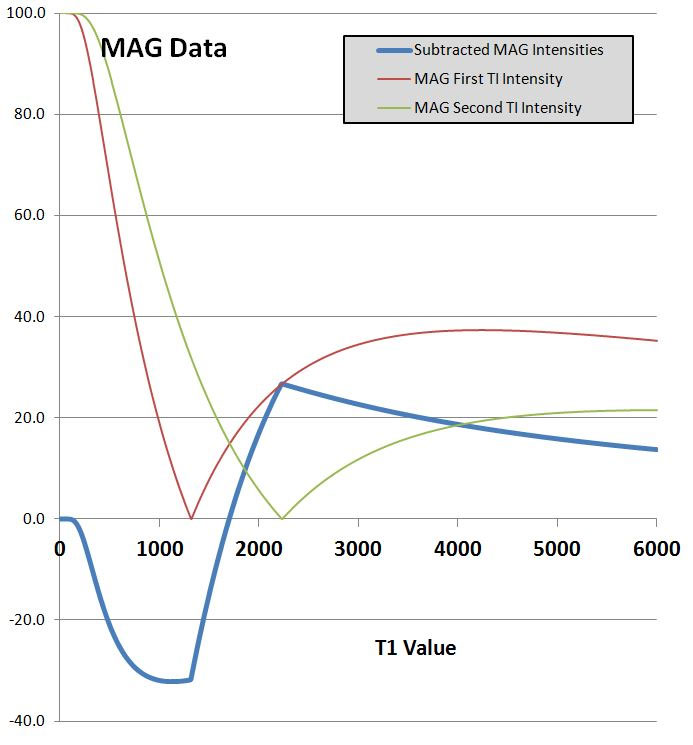
Fig. 11. Plot of signal versus T1 for magnitude reconstructed images with a short TI and for a longer TI, together with a subtraction (short TI image minus long TI image) shown in blue. The subtraction curve is a high pass filter with a steeply sloping transition band for T1s between the T1s corresponding to the null points of the shorter and longer TI sequences. This shows its very high sequence weighting. It results in very high positive contrast for increases in T1 in the range between the two null points such as between white and grey matter of the brain.
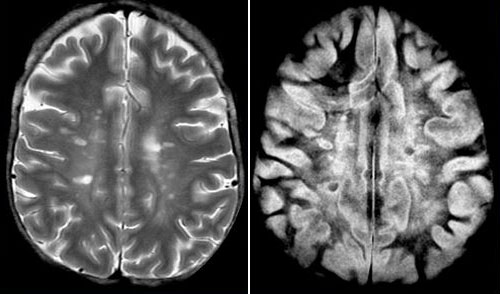
Fig. 12 (2018). Multiple sclerosis. T2-weighted SE image (A) and multiplied subtracted IR (MSIR) image (B). Discrete lesions are seen in the brain of the SE image in (A). These are seen in (B) with some having very low signal central regions. Much more extensive abnormality is seen in the central white matter in (B). In many areas this extends out towards the subcortical regions and only spares the U fibres. CSF is zero signal. The image in (B) provides evidence of very widespread abnormality in keeping with the modern understanding of the disease. The focal areas seen on the conventional T2-weighted SE image only represent a fraction of the underlying abnormality.
Conclusion
Ian's group was the first to demonstrate a decisive clinical advantage for MRI over state of the art CT (in 1981). This was crucial in facilitating the large scale investment in research and development necessary for the technique to achieve widespread routine clinical use.
Ian's group pioneered clinico-industrial collaborations which became the preferred mode of development for MRI. This was strongly supported by Surya Mohapatra of Picker/GEC. Those who did not establish arrangements of this type often lagged behind in the clinical arena.
Ian's basic approach to MRI of exploiting the technique's high soft tissue contrast and its many different MR tissue properties remains the basic strategy most used in clinical MRI today.
Before MR contrast agents became available, Ian's group found important clinical applications for MRI that did not require the use of them, and when these agents did become available for clinical use in 1984 they were the first group to describe their use.
An understanding of what clinicians wanted to see led to the development of the Multi Sequence Approach including one class of images that looked like classic anatomical sections of the brain, as well as another using heavily T2-weighted SE, STIR and FLAIR sequences which highlighted abnormalities in tissue due to disease.
After starting clinical work in 1981, by 1985 Ian and his group had written five of the ten most cited papers on clinical MRI. Two of the others were from the University of California, San Francisco (UCSF) with one each from the Mallinckrodt Institute of Radiology (St Louis), Case Western Reserve (Cleveland) and the Huntington Research Institute (Pasadena).
The group sustained low field imaging and kept it competitive with a series of technical developments including fat suppression with STIR when chemical shift techniques at higher fields were unreliable due to eddy current effects from non-shielded gradients. Low field imaging was taken up in Japan (which has over 50 MRI systems per million population compared with the 6-7 in the UK) and is being rediscovered in the US and Europe as "high performance, low field" imaging.
Ian was described as the Father of Clinical MRI. He and his group produced the first modern MR scanner (Fig. 4) which was very different from the laboratory prototypes previously used by groups working in MRI (e.g. Fig. 2). Contoured purpose designed coils which he developed (e.g. Fig. 7) are now a universal feature of MR systems. In addition, much of modern clinical brain and body imaging in adults and children uses artifact control methods, contrast agents and pulse sequences first demonstrated by Ian Young and his group. The pulse sequences include the T1-weighted IR, T2-weighted SE, T1 and heavily T2-weighted gradient echo and SWI, as well as STIR and FLAIR.
The disease most associated with MRI is MS, the appearances of which were first described by Ian in 1981 (5). James Prichard, neurologist at Yale said "Ian's early paper on MRI detection of clinically silent demyelinating lesions in MS was the earliest major and still one of the most important MRI discoveries pertinent to neurology. It showed that what we neurologists had always considered an unpredictable intermittent disease is actually a continuously progressive pathological condition of which only the clinical expression is intermittent. That insight both deepened our understanding of the basic processes at work in the disease and provided a new treatment evaluation metric that greatly sped up treatment research."
This was not achieved without a great deal of work from Ian McDonald, David Miller (both London), David Li, Donald Paty (both Vancouver), Massimo Filippi (Milan), Frederick Barkhoff (Amsterdam), Jack Simon (Denver), Douglas Arnold (Montreal), Paul Matthews (Montreal, Oxford, London), Joseph Frank (Bethesda), Bob Grossman (Philadelphia, New York) and numerous others.
Ian was appointed OBE in 1986 and elected FREng in 1988, and FRS in 1989. He received the Whittle medal from the Royal Academy of Engineering, and the Clifford Paterson medal from the Royal Society as well as honorary fellowships from the Royal College of Radiologists and the Royal College of Physicians. He was also awarded an honorary DSc by Aberdeen University.
Ian is survived by his wife Sylvia, their children Graham, Neil and Fiona, as well as six grandchildren.
References
1. Hounsfield GN. Computed Medical Imaging. Nobel lecture, December 8 1979. J Comput Assist Tomogr 1980;4:665-74.
2. Higson GR. Seeing things more clearly. Br J Radiol 1987;60:1049-57.
3. Wood A. Magnetic Venture: The Story of Oxford Instruments. Oxford: Oxford University Press, 2001.
4. Worthington BS, Isherwood I. In: Wellcome Witnesses to Twentieth Century Medicine, vol. 2. Making the Human Body Transparent: The Impact of Nuclear Magnetic Resonance and Magnetic Resonance Imaging. Eds: Tansey EM, Christie DA. London: The Trustees of the Wellcome Trust 1998 p. 32 and p. 59.
5. Young IR, Hall AS, Pallis CA, Legg NJ, Bydder GM, Steiner RE. Nuclear magnetic resonance of the brain in multiple sclerosis. Lancet 1981;ii:1063-66.
6. Abetti P, Haldar P. One hundred years of superconductivity: science, technology, products, profits and industry structures. In J Technology Management 2009;48:423-47.
7. Bailes DR, Young IR, Thomas DJ, Straughan K, Bydder GM, Steiner RE. NMR imaging of the brain using spin-echo sequences. Clin Radiol 1982;33:395-414.
8. Edelstein WA, Hutchison JM, Johnson G, Redpath T. Spin warp NMR imaging and applications to human whole-body imaging. Phys Med Biol 1980;25:751-56.
9. Young IR, Burl M, Clarke GJ, et al. Magnetic resonance properties of hydrogen: imaging the posterior fossa. AJR Am J Roentgenol 1981;137:895-901.
10. Young IR, Bailes DR, Burl M, et al. Initial clinical evaluation of a whole body nuclear magnetic resonance (NMR) tomograph. J Comput Assist 1982;6:1-18.
11. Hawkes RC, Holland GN, Moore WS, Worthington BS. Nuclear magnetic resonance (NMR) tomography of the brain: a preliminary clinical assessment with demonstration of pathology. J Comput Assist Tomogr 1980;4:677-86.
12. Bryant DJ, Payne JA, Firmin DN, Longmore DB. Measurement of flow with NMR imaging using a gradient pulse and phase difference technique. J Comput Assist Tomogr 1984;8:588-93.
13. Bydder GM, Young IR. MR imaging: clinical use of the inversion recovery sequence. J Comput Assist Tomogr 1985;9:242-51.
14. Bailes DR, Gilderdale DJ, Bydder GM, Collins AG, Firmin DN. Respiratory Ordered Phase Encoding (ROPE): a method for reducing respiratory motion artefacts in MR imaging. J Comput Assist Tomogr 1985;9:835-38.
15. Weinmann H-J, Brasch RC, Press WR, Wesbey GE. Characteristics of gadolinium-DTPA complex: a potential NMR contrast agent. AJR Am J Roentgenol 1984;142:619-24.
16. Carr DH, Brown J, Bydder GM, et al. Intravenous chelated gadolinium as a contrast agent in NMR imaging of cerebral tumours. Lancet 1984;i:484-86.
17. Bydder GM, Kingsley DP, Brown J, Niendorf HP, Young IR. MR imaging of meningiomas including studies with and without gadolinium-DTPA. J Comput Assist Tomogr 1985;9:690-97.
18. Mackay IM, Bydder GM, Young IR. MR imaging of central nervous system tumors that do not display in increase in T1 or T2. J Comput Assist Tomogr 1985;9:1055-61.
19. Gomori JM, Grossman RI, Goldsberg HI, Zimmerman RA, Bilaniuk LT. Intracranial hematomas: imaging by high-field MR. Radiology 1985;157:87-93.
20. Bydder GM, Pennock JM, Porteous R, et al. MRI of intracerebral haematoma at low field (0.15T) using T2 dependent partial saturation sequences. Neuroradiology 1988;30:367-71.
21. Young IR, Khenia S, Thomas DG, et al. Clinical magnetic susceptibility mapping of the brain. J Comput Assist Tomogr 1987;11:2-6.
22. Duyn J. MR susceptibility imaging. J Magn Reson 2013;229:198-207.
23. Hajnal JV, Bryant DJ, Kasuboski L, et al. Use of Fluid Attenuated Inversion Recovery (FLAIR) pulse sequences in MRI of the brain. J Comput Assist Tomogr 1992 16:841-44.
24. Marsh H. Admissions: A Life in Brain Surgery. New York: Thomas Dunne; 2017 pp 10-11
25. Levene MI, Whitelaw A, Dubowitz V, et al. Nuclear magnetic resonance imaging of the brain in children. Br Med J 1982;285(6344):774-76.
26. Hall AS, Young IR, Davies FJ, Mohapatra SN. A dedicated MR system in a neonatal intensive therapy unit. In: WG Bradley, GM Bydder, eds. Advanced MR Imaging Techniques. London: Martin Dunitz 1997 pp 281-289.
27. Battin MR, Maalouf EF, Counsell SJ, et al. Magnetic resonance imaging of the brain in very preterm infants: visualisation of the germinal matrix early myelination and cortical folding. Pediatrics 1998;101:957-62.
28. McGinley JV, Ristic M, Young IR. A permanent MRI magnet for magic angle imaging having its field parallel to the poles. J Magn Reson 2016;271:60-67.
29. Chappell KE, Brujic D, van Der Streeten C, et al. Detection of maturity and ligament injury using magic angle direction imaging. Magn Reson Med 2019;82:1041-54.
30. Axel L. Revised glossary of MR terms. Radiology 1987;162:874.
31. Redpath TW, Smith FW. Technical note: use of a double inversion recovery pulse sequence to image selectively grey or white brain matter. Br J Radiol 1994; 67:1258- 63.
The History of the Development of MRI and MRS in the UK (MRIS History UK) (https://mrishistory.org.uk):
32. Bryant DJ, Cox IJ, Taylor-Robinson SD. The Hammersmith spin on metabolism using clinical MR spectroscopy.
33. DeSouza NM, Gilderdale DJ. Endocavity and interventional magnetic resonance imaging (MRI) - the Hammersmith years.
34. Young IR. My involvement with MRI and MRS at EMI and Hammersmith Hospital.
35. Young IR. The Societies.
36. Bottomley PA. On the origins of localized NMR: view from an accomplice.
37. Hoult DI. My time in MRS and MRI at Oxford.
38. Gedroyc WM. A personal history of MRI at St Mary's.
39. Wardlaw JM. History of MRI/S in the UK.
40. Rinck PA. An excursion into the history of MR imaging.
41. Thomas AMK. NMR and MRI: historical reflections.
42. Griffiths JR. Involvement with SMRM and ISMRM.
43. Harrison C. Engineering early MRI systems.
44. Anand P. MRI.
45. McKinstry CS. Reminiscences of the MRI Unit at Hammersmith Hospital, 1985-86.
46. Syms R. Ian Young FRS.
47. Paley MN. A quick scan of my life with MRI and MRS in industry and academia.
48. Bydder GM. Clinical MRI and MRS at Hammersmith Hospital.

