Digital Poster
Managing Motion in the Brain & Body I
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
1950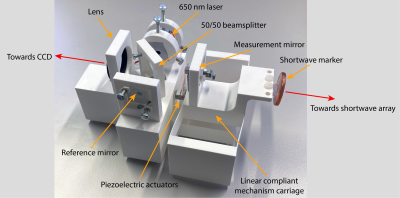 |
45 | Motion tracking verification with a 3D printed compliant mechanisms and laser interferometry
Christoph Michael Schildknecht1 and Klaas Paul Prüssmann1
1Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zürich, Switzerland
Precisely characterizing motion tracking modalities in the MRI bore is quite a challenge. Especially when not only static positions ought to be evaluated, but also dynamic characterization is desired. In this work, we show how a compliant mechanism in the form of a folded-beam suspension can be utilized for accurate dynamic positioning. A piezoelectric actuator drives the system, and position feedback is acquired with a laser interferometer. Testing the system with short-wave motion tracking revealed a sub-micrometre agreement.
|
||
1951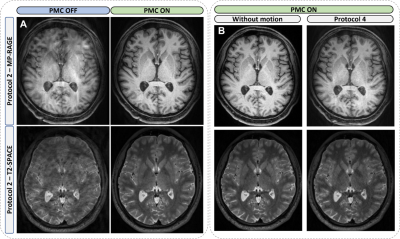 |
46 | Evaluation of markerless prospective motion correction for neuroanatomical MRI
Zakaria Zariry1,2, Robert Frost3,4, Franck Lamberton2,5, Thomas Troalen6, Nathalie Richard1,2, Andre van der Kouwe3,4, and Bassem Hiba1,2
1Institute of Cognitive Neuroscience Marc Jeannerod, CNRS / UMR 5229, BRON, France, Metropolitan, 2Université Claude Bernard - Lyon 1, Villeurbanne, France, Metropolitan, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5Cermep, CNRS / UAR 3453, Lyon, France, Metropolitan, 6Siemens Healthcare SAS, Saint-Denis, France, Metropolitan
Head-motion is a main cause of artifacts in MRI. The performance of a markerless optical system, which records the subject's face, estimates head-motion, and allows real-time repositioning of the FOV, is evaluated for neuroanatomical MRI. Sets of T1W/T2W-images were collected from 2 subjects instructed to perform different head-motion protocols during the acquisitions. The System performance is evaluated by comparing images with/without motion-correction. The optical system ensures good corrections of head-motions. However, the correction quality depends on the amplitude of the movement, its location in k-space and its nature. Residual effects of large amplitudes/amounts movements may persist on corrected images.
|
||
1952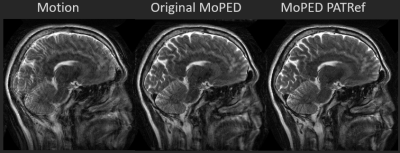 |
47 | Think outside the box: Exploiting the imaging workflow for Deep Learning based motion estimation and correction
Julian Hossbach1,2, Daniel Nicolas Splitthoff3, Bryan Clifford4, Daniel Polak3,5, Wei-Ching Lo4, Stephan Cauley5, and Andreas Maier1
1Pattern Recognition Lab Friedrich-Alexander-University Erlangen-Nuremberg, Erlangen, Germany, 2Erlangen Graduate School in Advanced Optical Technologies, Erlangen, Germany, 3Siemens Healthcare, Erlangen, Germany, 4Siemens Medical Solutions, Malden, MA, United States, 5Department of Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States Estimating intrascan subject motion enables the reduction of motion artifacts but often requires further calibration data e.g. from an additional motion-free reference. In this work, we explore how the reuse of supplementary scans in the imaging workflow can be used as motion calibration data. More specifically, the preceding parallel imaging calibration scan is reutilized to support a Deep Learning (DL) approach for estimating motion. Results are presented which indicate that DL, in contrast to a conventional optimization approach, can extract the motion and improve the image quality despite contrast differences between the calibration and imaging scans. |
||
1953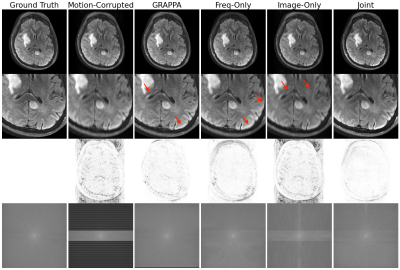 |
48 | Joint Neural Network for Fast Retrospective Rigid Motion Correction of Accelerated Segmented Multislice MRI
Nalini M Singh1,2, Malte Hoffmann3,4, Daniel C Moyer1, Ikbeom Jang3,4, Lina Chen5, Marcio Aloisio Bezerra Cavalcanti Rockenbach5, Arnaud Guidon6, Iman Aganj3,4, Jayashree Kalpathy-Cramer3,4,5, Elfar Adalsteinsson2,7,8, Bruce Fischl2,3,4, Adrian V Dalca1,3,4, Polina Golland*1,7,8, and Robert Frost*3,4
1Computer Science and Artificial Intelligence Laboratory, Massachusetts Institute of Technology, Cambridge, MA, United States, 2Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5MGH & BWH Center for Clinical Data Science, Mass General Brigham, Boston, MA, United States, 6GE Healthcare, Boston, MA, United States, 7Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 8Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, United States
We demonstrate a deep learning approach for fast retrospective intraslice rigid motion correction in segmented multislice MRI. The proposed neural network architecture combines convolutions on frequency and image space representations of the acquired data to produce high quality reconstructions. Unlike many prior techniques, our method does not require auxiliary information on the subject head motion during the scan. The resulting reconstruction procedure is more accurate and is an order of magnitude faster than GRAPPA. Our work offers the first step toward fast motion correction in any setting with substantial, unpredictable, challenging to track motion.
|
||
1954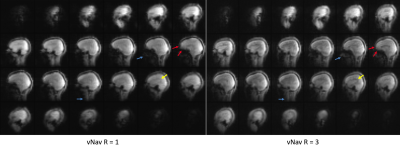 |
49 | Accelerated 3D EPI navigator for prospective motion correction Video Not Available
Yulin Chang1, Daniel Nicolas Splitthoff2, Wei-ching Lo1, M. Dylan Tisdall3, and Andre van der Kouwe4
1Siemens Medical Solutions USA Inc., Malvern, PA, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Radiology, University of Pennsylvania, Philadelphia, PA, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Harvard Medical School, Massachusetts General Hospital, Boston, MA, United States
We show that for navigator-based prospective motion correction MRI, acceleration of 3D EPI acquisition increases sequence flexibility and improves the navigator image quality without sacrificing the quality of motion correction.
|
||
1955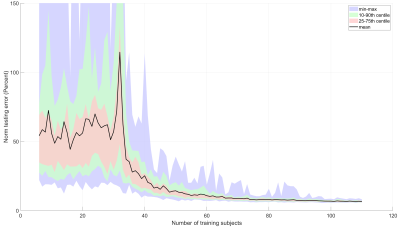 |
50 | Pilot-Tone Motion Estimation for Brain Imaging at Ultra-High Field Using FatNav Calibration
Tom Wilkinson1,2, Yannick Brackenier1,2, Felipe Godinez3, Raphael Tomi-Tricot1,2,4, Jan Sedlacik1,2, Philippa Bridgen1,2, Sharon Giles1,2, Joseph V Hajnal1,2, and Shaihan J Malik1,2
1Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2London Collaborative Ultra high field System (LoCUS), London, United Kingdom, 3Department of Radiology, University of California Davis, Sacramento, CA, United States, 4MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
3D FatNavs were used to provide a rapid, robust pre-scan to calibrate motion estimation from pilot-tone. The amount of training data required to make reliable forward predictions was investigated. This method robustly predicts motion within a dataset and can be applied to other datasets with lower accuracy. Improving the accuracy and speed of this method is ongoing work. This independent method of motion estimation shows promise for rapid calibration as part of routine examinations and paves the way to offer motion correction at ultra-high field where motion-correction is particularly relevant.
|
||
1956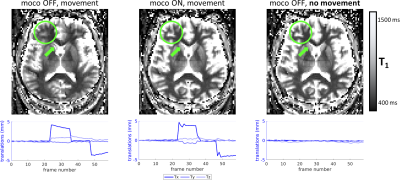 |
51 | Prospective motion correction in multi-inversion EPI using volumetric navigators for robust T1 map estimation
Jonathan R. Polimeni1,2,3, M. Dylan Tisdall4, Daniel J. Park1, Paul Wighton1, S. Robert Frost1,2, Christine L. Tardif5,6, and Andre J. W. van der Kouwe1,2
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 5Department of Biomedical Engineering, Department of Neurology & Neurosurgery, McGill University, Montreal, QC, Canada, 6McConnell Brain Imaging Centre, Montreal Neurological Institute, Montreal, QC, Canada
In multi-inversion EPI (MI-EPI), each slice samples a distinct inversion time during each inversion recovery, providing an efficient method for estimating T1. MI-EPI is vulnerable to through-plane motion, which results in slices sampling a subset of the desired inversion times and wrong TIs will be attributed to the slices. This cannot be corrected retrospectively. We introduce prospective motion correction in MI-EPI using volumetric navigators (vNavs). vNavs are acquired at the beginning of the inversion recovery thus the effects of their excitation pulses must be modeled for T1 estimation. This provides improved T1 estimation accuracy in the presence of subject motion.
|
||
1957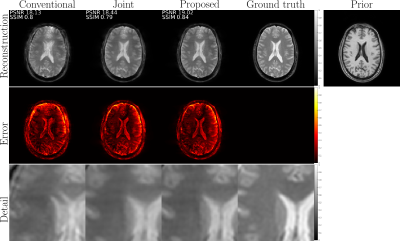 |
52 | Retrospective motion correction with structural priors for clinical MRI protocols
Gabrio Rizzuti1, Niek Huttinga2, Alessandro Sbrizzi2, and Tristan van Leeuwen3
1Utrecht University, Utrecht, Netherlands, 2Universitair Medisch Centrum Utrecht, Utrecht, Netherlands, 3Centrum Wiskunde & Informatica, Amsterdam, Netherlands
We present a retrospective rigid motion correction and reconstruction scheme for brain MRI with the aid of structural priors. The proposed framework is designed to be applied to a clinical protocol including multiple scans for multiple contrasts, of which some scans are corrupted due to motion during the acquisitions, but at least one is uncorrupted and exploited as an additional prior. We envision a practical workflow that can easily fit into the existing clinical practice without the need for changing the MRI acquisition protocols.
|
||
1958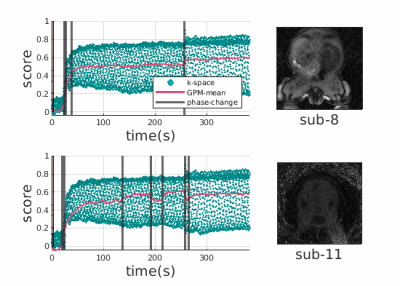 |
53 | Gaussian Process Modelling and Compensation of Motion in DCE-MRI
Aziz Kocanaogullari1, Cemre Ariyurek1, Onur Afacan1, and Sila Kurugol1
1Radiology, Boston Children's Hospital, Boston, MA, United States
Radial DCE-MRI is robust to motion. However, bulk motion or heavy breathing causes 1) irrecoverably deteriorated k-space lines acquired during motion events reducing image quality, 2) misaligned volumes in a dynamic sequence. In this work we propose to solve the first problem by fitting a Gaussian process to the k-space center of each spoke over time and using it to determine outlier spokes corrupted by motion. We solve the second problem by clustering the dynamic data to respective motionless phases before and after each motion event and registering volumes between phases for computationally efficient correction of motion with fewer registrations.
|
||
1959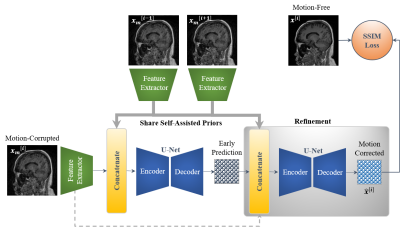 |
54 | Self-Assisted Priors with Cascaded Refinement Network for Reduction of Rigid Motion Artifacts in Brain MRI
Mohammed A. Al-masni1, Seul Lee1, Jaeuk Yi1, Sewook Kim1, Sung-Min Gho2, Young Hun Choi3, and Dong-Hyun Kim1
1Department of Electrical and Electronic Engineering, College of Engineering, Yonsei University, Seoul, Korea, Republic of, 2GE Healthcare, Seoul, Korea, Republic of, 3Department of Radiology, Seoul National University Hospital, Seoul, Korea, Republic of
MRI is sensitive to motion caused by patient movement. It may cause severe degradation of image quality. We develop an efficient retrospective deep learning method called stacked U-Nets with self-assisted priors to reduce the rigid motion artifacts in MRI. The proposed work exploits the usage of additional knowledge priors from the corrupted images themselves without the need for additional contrast data. We further design a refinement stacked U-Nets that facilitates preserving of the image spatial details and hence improves the pixel-to-pixel dependency. The experimental results prove the feasibility of self-assisted priors since it does not require any further data scans.
|
||
1960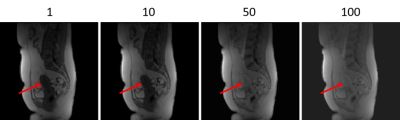 |
55 | Registration of abdominal DCE-MRI: optimal choice of reference and moving image pairs
Adam George Tattersall1,2, Keith A Goatman2, Scott Semple1, and Lucy E Kershaw1
1University of Edinburgh, Edinburgh, United Kingdom, 2Canon Medical Research Europe, Edinburgh, United Kingdom
Before registration can take place, an approach to pairing images must be chosen. We used synthetic data to provide a ground truth to evaluate four approaches to pairing images. Our synthetic data was created using tracer kinetics modelling of abdominal DCE-MRI to create motionless data. Then local distortions were applied to the motionless data. We found that using one image as a reference image produced the best result to eliminate the propagation of errors. The reference image must have a similar intensity distribution to the other images in the sequence.
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.