Digital Poster
Cardiac Anatomy & Tissue Characterization I
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
1002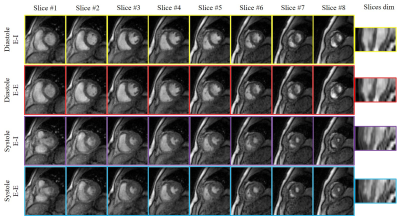 |
9 | Alignment and joint recovery of multi-slice free-breathing cardiac cine using manifold learning
Qing Zou1, Abdul Haseeb Ahmed1, Prashant Nagpal2, Sarv Priya1, Rolf F. Schulte3, and Mathews Jacob1
1University of Iowa, Iowa City, IA, United States, 2University of Wisconsin–Madison, Madison, WI, United States, 3GE Global Research, Munich, Germany
Free-breathing cardiac cine methods are needed for pediatric and chronic obstructive pulmonary disease (COPD) subjects. Multi-slice acquisitions can offer good blood-myocardium contrast, and hence preferred over 3D methods. Current approaches independently recover the slices, followed by post-processing to combine data from different slices. In this work, a deep manifold learning scheme is introduced for the joint alignment and reconstruction of multi-slice dynamic MRI. The proposed scheme jointly learns the parameters of the deep network as well as the latent vectors for each slice, which captures the motion induced dynamic variations, from the k-t space data of the specific subject.
|
||
1003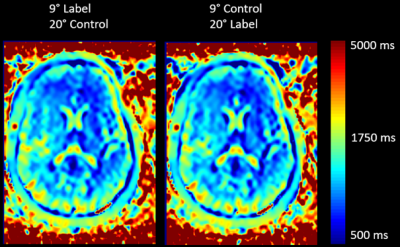 |
10 | Individualized arterial spin labeling background suppression by rapid T1 mapping during acquisition
Thomas Lindner1, Friederike Austein1, Helena Guerreiro1, and Jens fiehler1
1Department of Diagnostic and Interventional Neuroradiology, University Hospital Hamburg-Eppendorf, Hamburg, Germany
This work presents a method of rapidly acquiring a T1 map to optimize background suppression in Arterial Spin Labeling to improve the signal-to-background contrast.
|
||
1004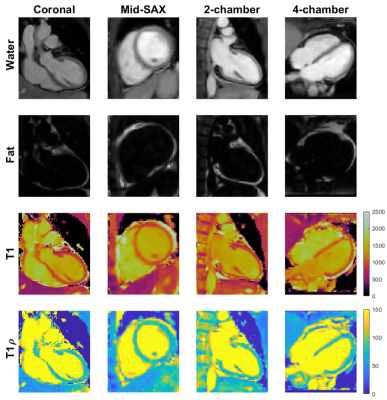 |
11 | 3D joint T1/T1ρ mapping and water-fat imaging for contrast-agent free myocardial tissue characterization
Michael G Crabb1, Karl P Kunze1,2, Carlos Velasco1, Anastasia Fotaki1, Camila Munoz1, Alina Hua1, Radhouene Neji1,2, Claudia Prieto1, and Rene M Botnar1
1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
In patients with suspected myocardial infarction, late-gadolinium enhancement (LGE) images are often acquired to detect focal and diffuse scarring. T1ρ is a sensitive marker for assessment of macromolecular-water interaction and has shown potential for non-contrast enhanced imaging of fibrosis. Here we propose a novel free-breathing, 3D whole-heart joint T1/T1ρ mapping sequence with Dixon encoding to provide 3D T1 and T1ρ maps and co-registered water and fat volumes with isotropic resolution for comprehensive contrast-agent free myocardial tissue characterization. Preliminary 3D joint T1/T1ρ mapping and water/fat imaging results demonstrate good agreement in phantoms and promising results in-vivo in healthy volunteers and patients.
|
||
 |
1005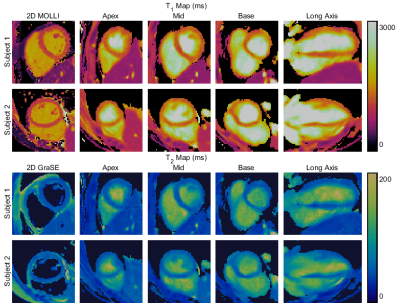 |
12 | Free-running 3D T1 and T2 myocardial mapping and cine MRI in 3 mins using low-rank non-rigid motion-corrected reconstruction
Andrew Phair1, Gastao Cruz1, Haikun Qi2, René Botnar1, and Claudia Prieto1
1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2School of Biomedical Engineering, Shanghai Tech University, Shanghai, China
Recent work has enabled the simultaneous acquisition of 3D myocardial T1 and T2 maps with isotropic spatial resolution and cardiac cine images from a ~10-minute scan. Herein, we propose to incorporate non-rigid cardiac motion correction into a dictionary-based low-rank reconstruction scheme, allowing k-space data from all cardiac phases to be included in the reconstruction of any given phase. Reconstructed cine images and T1 and T2 maps with and without motion correction are presented and demonstrate that a reduction to 30% of the acquired k-space data (~3-minute scan) can be achieved while image quality is maintained.
|
|
1006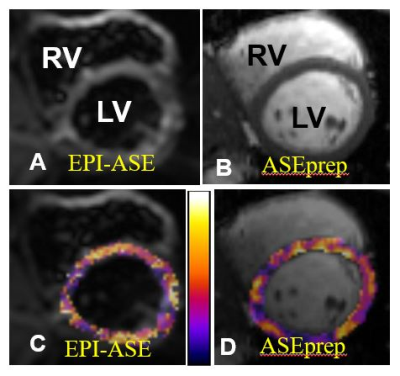 |
13 | A New MRI acquisition method for quantitative myocardial oxygen extraction imaging
Jie Zheng1,2, Ran Li1,2, Cihat Eldeniz1, Thomas H Schindler1, Linda R Peterson3, and Pamela K Woodard1,2
1Mallinckrodt Institute of Radiology, Washington University in St. Louis, Saint Louis, MO, United States, 2Department of Biomedical Engineering, Washington University in St. Louis, Saint Louis, MO, United States, 3Department of Medicine, Cardiovascular Division, Washington University in St. Louis, Saint Louis, MO, United States
A previously developed MRI method for quantitative myocardial oxygen extraction mapping showed promising results, but image quality suffered from distortion and low in-plane resolution. Therefore we developed a new image acquisition method which both doubled the in-plane spatial resolution and corrected image distortion and tested it in healthy subjects. Reproducibility studies showed comparable results between the two methods. Rigorous animal and/or human validation studies are warranted to study its translational potential in the assessment of patients with myocardial metabolic dysfunction.
|
||
1007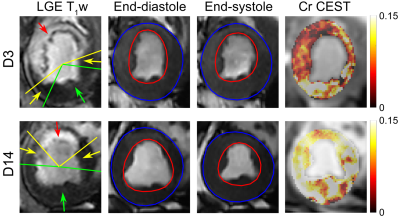 |
14 | MR chemical exchange saturation transfer study of myocardium creatine dynamic change in an acute infarct porcine model
Chuan-Miao Xie1, Jie Liu2, Jia-Lei Zhao2, Qi Liu3, Jian Xu3, Li-Yun Zheng4, Xin Liu2, Hairong Zheng2, and Yin Wu2
1Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China, 2Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 3UIH America, Inc., Houston, TX, United States, 4Central Research Institute, United Imaging Healthcare, Shanghai, China This study investigated the dynamic alteration of myocardium creatine in infarct hearts using CEST MRI. Seven pigs underwent cine, Cr CEST and LGE imaging 3 and 14 days after MI. Significant increase of stroke volume and ejection fraction and decrease of infarct angle were observed at day 14 compared to that at day 3. Meanwhile, Cr CEST signals increased significantly in the infarct and its adjacent myocardium, and negatively correlated with infarct angle. The improved metabolic activity was associated with function recovery, indicating that Cr CEST MRI is promising to provide complementary information for heart remodeling at the molecular level. |
||
1008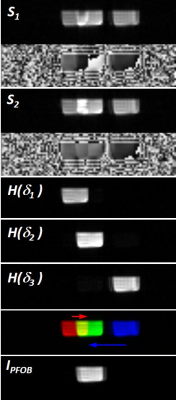 |
15 | Chemical Shift Artifact Correction for 19F MRI using Spectral Hadamard Encoding Video Permission Withheld
Michael Bock1, Serhat Ilbey1, Alexander Maier2, and Ali Caglar Özen1
1Radiology - Medical Physics, University Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2Cardiology and Angiology I, University Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Chemical shift artifact correction is achieved via spectral Hadamard encoding of the individual resonances using composite RF pulses. During decoding individual images of the resonances are created that can be combined to a final composite image. The method is demonstrated in 19F MRI of perfluorooctyl bromide that can be used to label monocytes for inflammation. Compared to other correction methods Hadamard decoding can be implemented directly in the image reconstruction, and the full SNR advantage of the image combination can be realized.
|
||
1009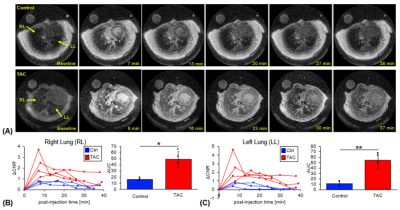 |
16 | Dynamic UTE molecular MR imaging targeting pulmonary fibrogenesis in a model of left ventricular dysfunction
Brianna F. Moon1,2, Iris Y. Zhou1,2, Yingying Ning1,2, Sergey Shuvaev1,2, Mariane M. Le Fur1,2, Yin-Ching I. Chen1, Eman A. Akam3, Jonah P. Weigand-Whittier1, Nicholas Rotile1,2, Matthew Drummond4, Avery T. Boice1,2, Samantha E. Zygmont1,2, Rod R. Warburton5, Barry L. Fanburg5, Krishna C. Penumatsa5, and Peter D. Caravan1,2
1Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Radiology, Institute for Innovation in Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 3Medicine, Division of Cardiology, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 4Medicine, Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 5Pulmonary, Critical Care and Sleep Medicine, Tufts Medical Center, Boston, MA, United States Group 2 pulmonary hypertension is a complication of chronic left ventricular dysfunction. During the early stages of the disease activity there is an accumulation of extracellular matrix molecules and an increase in allysine content. Molecular MR imaging with an allysine-targeted fibrogenesis probe shows increased lung-to-muscle ΔCNR within the lungs of transverse aortic constriction (TAC)-induced left ventricular dysfunction animal models compared to control animals, which corresponded to increased lung and right ventricle weight, and elevation of fibrogenesis biomarkers (hydroxyproline and allysine). |
||
1010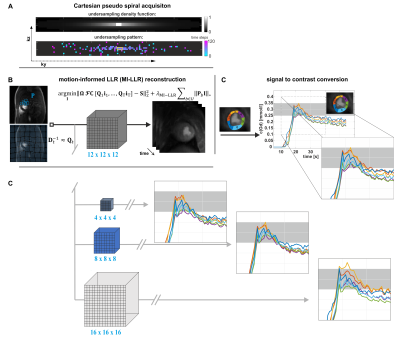 |
17 | Performance of Quantitative, Free-breathing 3D Perfusion CMR using Motion-Informed LLR Reconstruction in a Porcine Infarction Model
Tobias Hoh1, Valery Vishnevskiy1, Christian T Stoeck1,2, Conny F Waschkies2, Nikola Cesarovic3, Miriam Weisskopf2, Maximilian Fuetterer1, and Sebastian Kozerke1
1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland, 2Center of Surgical Research, University Hospital Zurich, University of Zurich, Zurich, Switzerland, 3Department of Health Sciences and Technology, ETH Zurich, Zurich, Switzerland
Three-dimensional first-pass myocardial perfusion CMR requires acceleration methods to enable whole-heart coverage in a limited acquisition window. Current 3D approaches suffer from data inconsistencies during free breathing, compromising image quality and perfusion quantification. We propose and experimentally validate a combination of Cartesian pseudo-spiral k-t undersampling with respiratory motion-informed locally low-rank (MI-LLR) reconstruction for robust whole-heart myocardial quantitative perfusion CMR in an experimental pig model of myocardial infarction. As reference, standard 2D acquisitions are used. Overall, image quality scoring was good. Perfusion defects were equally well depicted and discernible in imaging and MBF perfusion mapping in 3D and 2D approaches, respectively.
|
||
1011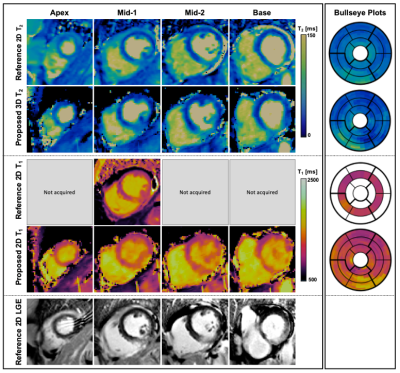 |
18 | Clinical evaluation and reproducibility of 3D whole-heart free-breathing joint T1/T2 quantification with isotropic resolution.
Carlos Velasco1, Alina Hua1, Anastasia Fotaki1, Camila Munoz1, Giorgia Milotta1, Karl P. Kunze1,2, Radhouene Neji1,2, Tevfik F. Ismail1, Claudia Prieto1, and Rene M. Botnar1
1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Myocardial tissue characterization including quantification of fibrosis and oedema plays an important role in the evaluation of many myocardial diseases. T1 and T2 maps are typically acquired sequentially in 2D under several breath-holds. However, misregistration artifacts, spatial resolution and volumetric coverage remain a limitation. To address this, a high resolution, 3D whole-heart motion-compensated joint T1/T2 water/fat sequence has been proposed and validated in a phantom and in healthy subjects. Here, we demonstrate the feasibility of the proposed approach to simultaneously acquire whole-heart co-registered T1, T2 maps as well as water and fat volumes in ~9min in patients with cardiovascular disease.
|
||
1012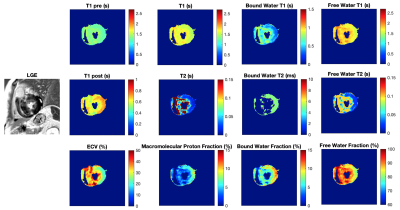 |
19 | MultI-Compartment Model based Parametric Mapping of the Heart with a Single MOLLI Acquisition (MIC-MOLLI) Video Permission Withheld
Jing Liu1, Karen Ordovas2, Yan Wang1, Janine Lupo1, Duan Xu1, Yoojin Lee1, Roselle Abraham1, and David Saloner1
1University of California San Francisco, San Francisco, CA, United States, 2University of Washington, Seattle, WA, United States
In this study, we developed a multi-compartment model based method for deriving multi-parametric mapping of the heart from the MOLLI T1 mapping acquisition. The preliminary results on patients with hypertrophic cardiomyopathy demonstrated the feasibility of deriving 9 quantitative parametric maps from a single scan and showed its potential of providing a comprehensive assessment of the myocardial tissue composition.
|
||
1013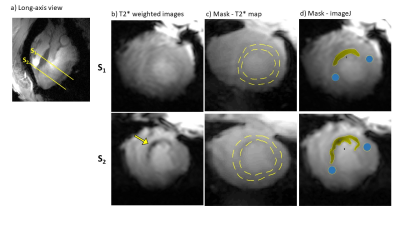 |
20 | Comparison of T2*-weighted image analysis with quantitative T2* maps in different stages of myocardial infarction in a pig model study with 7T cMRI
Julia Aures1, Maxim Terekhov1, David Lohr1, Maya Bille1, Michael Hock1, Ibrahim Elabyad1, Florian Schnitter2, Wolfgang Bauer1,2, Ulrich Hofmann2, and Laura Schreiber1
1Chair of Molecular and Cellular Imaging, Comprehensive Heart Failure Center, University Hospital Wuerzburg, Wuerzburg, Germany, 2Department of Internal Medicine I, Cardiology, University Hospital Wuerzburg, Wuerzburg, Germany
In the field of ultra-high field MRI, T2* mapping is a promising technique for the non-invasive assessment of myocardial pathophysiology. Preclinical studies have already shown the potential to detect structural changes in infarcted myocardial tissue. Quantitative T2* imaging is very demanding with regard to measurement- and postprocessing time. Hence, we compared in this study the simpler and faster grayscale analysis of T2*-weighted images with quantitative T2* techniques. This was done in early and late acute infarct healing stages in a large animal model by comparing T2* maps with grayscale T2*-weighted images.
|
||
1014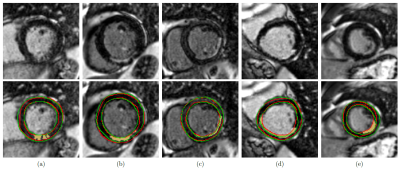 |
21 | Improving the Sensitivity to Myocardial Scar in Automatic Segmentations of Left Ventricular Myocardium on LGE Images
Marc Vornehm1,2, Maximilian Fenski3, Elisabeth Preuhs4, Andreas Maier4, Jeanette Schulz-Menger3, Jens Wetzl1, and Daniel Giese1,5
1Magnetic Resonance, Siemens Healthcare GmbH, Erlangen, Germany, 2Artificial Intelligence in Biomedical Engineering, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 3Working Group Cardiovascular Magnetic Resonance, Experimental and Clinical Research Center, Charité Medical Faculty, Max Delbrück Center for Molecular Medicine, HELIOS Klinikum Berlin Buch, Department of Cardiology and Nephrology, Berlin, Germany, 4Pattern Recognition Lab, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 5Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
Automatic segmentation of left ventricular myocardium on LGE images of patients with chronic myocardial infarction is challenging due to inhomogeneous signal intensities in the presence of myocardial scar. However, the inclusion of scar in automatically generated myocardium segmentations is critical for further LGE analysis. We propose a Deep Learning-based method for myocardial segmentation on cardiac LGE images, achieving average Dice scores of 0.78 and 0.80 on test images with and without scar, respectively. We furthermore evaluate the network’s sensitivity to scar and show that it can be improved by incorporating synthetic images with diverse enhancement patterns in the training data.
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.