Digital Poster
Cardiac Anatomy & Tissue Characterization II
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
1107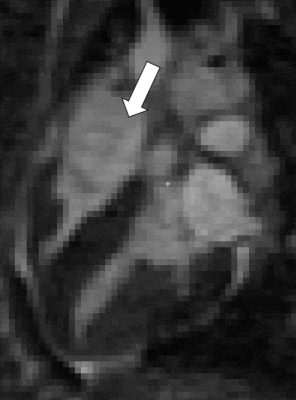 |
12 | Dynamic Pressure-Volume Loop Analysis using Simultaneous Real-Time Cardiac MRI and Left Heart Catheterization
Felicia Seemann1, Christopher G Bruce1, Jaffar M Khan1, Rajiv Ramasawmy1, Amanda Potersnak1, Daniel A Herzka1, Andi Jaimes1, William H. Schenke1, Kendall O’Brien1, Robert J Lederman1, and Adrienne E Campbell-Washburn1
1Cardiovascular Branch, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States
Pressure-volume (PV) loops can be used to derive the valuable cardiac functional parameters contractility and compliance. Conventional PV loop catheters provide unreliable volume measurements. In this study we develop and validate a method to obtain dynamic PV loops during an MRI-guided preload alteration via inferior vena cava occlusion, using simultaneous measurements of left ventricular pressure from a catheter and volume from real-time long-axis cardiac MRI. Pressure and volume signals were synchronized and used to derive contractility and compliance. Long-axis derived volumes were validated against conventional short-axis images. The method was tested in naïve, ischemic cardiomyopathy, and aortic banding swine models.
|
||
1108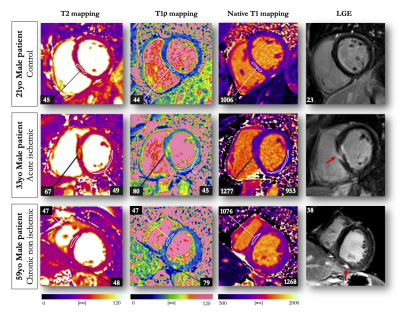 |
13 | Endogenous assessment of myocardial injuries using T1-rho mapping: comparison to T1 mapping, T2 mapping and late gadolinium enhancement imaging
Aurelien Bustin1,2,3, Xavier Pineau2, Soumaya Sridi2, Pierre Jaïs1,4, Matthias Stuber1,3,5, and Hubert Cochet1,2
1IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux – INSERM U1045, Bordeaux, France, 2Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 3Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4Department of Cardiac Electrophysiology, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 5CIBM Center for Biomedical Imaging, Lausanne, Switzerland
Myocardial T1-rho (T1ρ) mapping is a promising technique which reveals new insights about the macromolecular content of biological tissues. The idea that T1ρ mapping can be used to quantify myocardial fibrosis without contrast agent opens the door to new clinical capabilities. Yet, the tissue determinants driving T1ρ changes are still unclear, and the applicability of the technique to the broad spectrum of myocardial injuries remains uncharted territory. In the present study, we aim to identify clinical correlates of myocardial T1ρ and to examine how myocardial T1ρ values change under various clinical scenarios.
|
||
1109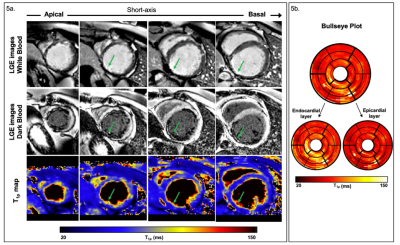 |
14 | 3D whole-heart free-breathing T1⍴ quantification: a preliminary clinical evaluation.
Carlos Velasco1, Anastasia Fotaki1, Haikun Qi1, Rene M. Botnar1, and Claudia Prieto1
1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
T1⍴-CMR allows the detection of scarred myocardial tissue without the need of an exogenous contrast. However, the generation of a single-slice T1ρ map requires sequential acquisitions under several breath-holds to acquire the T1⍴-weighted images at different spin-lock times, leading to long, inefficient scan times. We recently proposed a free-breathing motion-compensated 3D whole heart T1ρ mapping technique with near-isotropic spatial resolution (1.7×1.7×2 mm3) and predictable and clinically feasible scan In this work time (~8min). Here, we extend this technique to incorporate non-rigid motion corrected reconstruction and validate the proposed technique on subjects with suspected myocardial infarction.
|
||
1110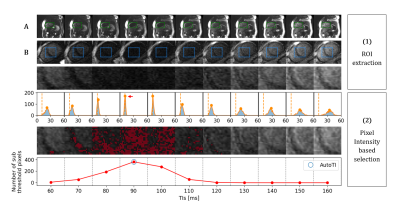 |
15 | Automated black-blood late gadolinium enhancement cardiac imaging through explainable user-independent inversion time selection
Aurelien Maillot1, Soumaya Sridi2, Xavier Pineau2, Amandine André-Billeau2, Stéphanie Hosteins2, Marta Nuñez-Garcia1, Maxime Sermesant1,3, Hubert Cochet1,2, Matthias Stuber1,4,5, and Aurelien Bustin1,2,4
1IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux – INSERM U1045, Bordeaux, France, 2Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 3INRIA, Université Côte d’Azur, Sophia Antipolis, France, 4Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 5CIBM Center for Biomedical Imaging, Lausanne, Switzerland
Black-blood late gadolinium enhancement (LGE) imaging techniques have been introduced to improve the poor scar to blood contrast of bright-blood LGE, especially for subendocardial myocardial infarction. These techniques heavily rely on the manual selection of the optimal inversion time (TI) for blood nulling which makes them operator-dependent, reduce their reproducibility and decrease clinical workflow efficiency. In this work, we investigate whether an explainable image processing technique can be employed for automated TI selection to enable fully automated black-blood LGE.
|
||
1111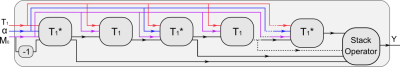 |
16 | Model-Based Reconstruction with Automatic Differentiation for Single-Shot Myocardial T1 Mapping using Radial MOLLI with FLASH Readout
Xiaoqing Wang1,2, Moritz Blumenthal1, Nick Scholand1,2, and Martin Uecker1,2,3
1Institute for Diagnostic and Interventional Radiology, University Medical Center Göttingen, Göttingen, Germany, 2German Centre for Cardiovascular Research (DZHK), Partner Site Göttingen, Göttingen, Germany, 3Institute of Medical Engineering, Graz University of Technology, Graz, Austria
A nonlinear operator for radial MOLLI is constructed in BART, allowing accurate derivative calculation via automatic differentiation. The constructed nonlinear MOLLI operator is further integrated into a model-based reconstruction framework where myocardial $$$T_{1}$$$ maps are reconstructed directly from undersampled k-space data. Results on an experimental phantom and one healthy subject have demonstrated that high resolution ($$$1.0 \times 1.0 \times 6$$$ mm$$$^{3}$$$) myocardial $$$T_{1}$$$ maps can be achieved within 4 heartbeats with good accuracy and precision using the proposed method.
|
||
1112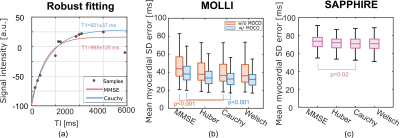 |
17 | M-Estimator for Robust Parameter Fitting in Quantitative Cardiac T1 Mapping Video Not Available
Yidong Zhao1, Changchun Yang1, Lu Huang2, Liming Xia2, Sebastian Weingärtner1, and Qian Tao1
1Department of Imaging Physics, Technische Universiteit Delft, Delft, Netherlands, 2Department of Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Quantitative cardiac T1 mapping involves nonlinear parameter estimation after MR acquisition. The widely used minimum mean square error (MMSE) estimator assumes Gaussian additive noise, and can be sensitive to outliers of non-Gaussian nature, such as those caused by cardiac motion. In this work, we propose to apply robust loss functions, which are part of the M-estimator family, with increased robustness to outliers. Experiments on MOLLI and SAPPHIRE sequences showed that the M-estimators were able to improve the T1 estimation robustness, significantly reducing the standard deviation (SD) error of the estimated T1 map in comparison to MMSE.
|
||
1113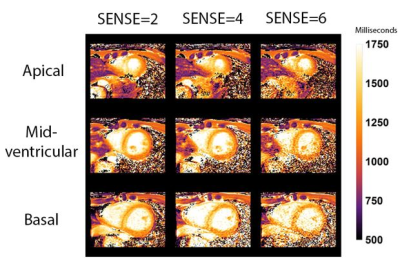 |
18 | Single breath-hold native myocardial T1 and T2 mapping using SENSE and a 72-channel cardiac receive array.
Hugo Klarenberg1, Mark Gosselink2, Tim Leiner2, Bram F. Coolen1, Aart J. Nederveen3, Adrianus J. Bakermans3, Hildo J. Lamb4, S. Matthijs Boekholdt5, Gustav J. Strijkers1, and Martijn Froeling2
1Physics & Bioengineering, Amsterdam UMC, Amsterdam, Netherlands, 2Radiology, University Medical Center Utrecht, Utrecht, Netherlands, 3Department of Radiology and Nuclear Medicine, Amsterdam UMC, Amsterdam, Netherlands, 4Radiology, Leiden University Medical Center, Leiden, Netherlands, 5Cardiology, Amsterdam UMC, Amsterdam, Netherlands
Single breath-hold native MOLLI T1 mapping in 3 slices is possible using SENSE=4 & 6 and a 72 channel receive array. T1 values in 16 segments of the 17-segment AHA model (excluding the apical segment) were similar compared to SENSE=2 using 3 breath-holds measured in 20 healthy subjects (10 female). Myocardial T2 GRaSE mapping with fewer breath-holds in 3 slices is possible, though at the expense of decreased accuracy of the T2 values in specific segments.
|
||
1114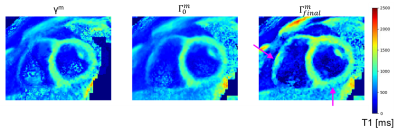 |
19 | 3D super-resolution motion-corrected cardiac T1 mapping
Simone Hufnagel1, Selma Metzner1, Christoph Stefan Aigner1, Jeanette Schulz-Menger2,3,4, Tobias Schaeffter1,5,6, and Christoph Kolbitsch1
1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2Charité Medical Faculty University Medicine, Berlin, Germany, 3Working Group on Cardiovascular Magnetic Resonance, Experimental and Clinical Research Center (ECRC), DZHK partner site Berlin, Berlin, Germany, 4Department of Cardiology and Nephrology, HELIOS Klinikum Berlin Buch, Berlin, Germany, 5School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 6Department of Biomedical Engineering, Technical University of Berlin, Berlin, Germany Cardiac T1 mapping provides valuable quantitative information about fibrosis in various cardiac diseases. Due to SNR limitations and the motion of the heart during imaging, often 2D T1 Maps with only low through-plane resolution (i.e. slice thickness of 6-8 mm) can be obtained. We present a model-based super-resolution reconstruction which combines multiple stacks of 2D acquisitions with 6 mm slice thickness and generates 3D high-resolution T1 maps. Cardiac and residual respiratory motion is corrected for. The approach was evaluated in native T1 mapping in three healthy volunteers and provided precise T1 maps with improved visualization of small structures. |
||
1115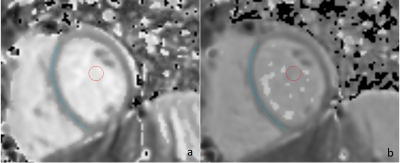 |
20 | The importance of optimized post-processing for accurate T1-mapping using shMOLLI: A phantom and in vivo evaluation at 3 T Video Permission Withheld
Linnéa Andersson1, Pär-Arne Svensson2, Charlotte De Lange2, and Kerstin Lagerstrand1,3
1Department of Medical Physics and Biomedical Engineering, Sahlgrenska University Hospital, Gothenburg, Sweden, 2Department of Paediatric Radiology, Queen Silvia Children's hospital, Sahlgrenska University Hospital, Gothenburg, Sweden, 3Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
We demonstrate the effect of not having relevant post-processing algorithm implemented when using the shMOLLI scan sequence for T1-mapping of myocardium and propose an optimization strategy where we use only the five first acquired images in post-processing. Phantom and in vivo measurements of T1-values in myocardium and blood pool in a pediatric population show the importance of using relevant post-processing: a large heart rate dependence is introduced in estimated T1-values without it. This heart rate dependency can, however, be effectively reduced by using only the five first acquired images in post-processing, resulting in measurements with high accuracy.
|
||
1116 |
21 | Consistent and Accurate 3T Cardiac End-Systolic Adiabatic T2-Mapping Video Permission Withheld
Ronald J Beyers1 and Thomas Denney1
1MRI Research Center, Auburn University, Auburn University, AL, United States
Cardiac T2-mapping is ideal for assessing myocardial edema resulting from events, such as, acute myocardial infarction, myocarditis and tako-tsubo cardiomyopathy. While many T2mapping sequences exist, most have design shortcuts that degrade their consistency, accuracy and prognostic value. T2maps captured at end-systole (ES) have maximum wall thickness and fewest artifacts and 4-point T2 curve-fits provide good T2 estimates. We present an Adiabatic T2-Prep Mapping sequence that produces accurate 4-point T2maps at ES within a 22-second breath hold at 3T. Pretesting on phantoms and in vivo validation on healthy human volunteers presented superior results.
|
||
1117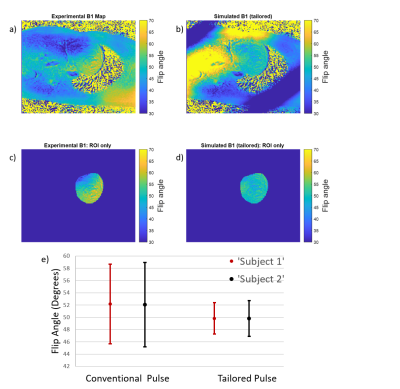 |
22 | Investigation of Spatial-Spectral Selective Pulses for B1 Compensation and Improved Saturation Homogeneity in Cardiac CEST MRI
Cindy Ayala1, Huiwen Luo2, Kevin Godines1, William A. Grissom2, and Moriel Vandsburger1
1Department of Bioengineering, University of California, Berkeley, Berkeley, CA, United States, 2Biomedical Engineering, Vanderbilt University, Nashville, TN, United States Chemical Exchange Saturation Transfer (CEST) MRI is highly susceptible to B1 inhomogeneities, resulting in inconsistent contrast for tissues with equal metabolite concentrations. To enhance B1-uniformity of saturation across the myocardium, a spectral-spatially selective pulse for CEST preparation was tailored for each subject. Simulations showed the tailored pulse reduced B1 variation from 6.85⁰ to 2.9⁰. These results were investigated on a Siemens 3T Trio scanner by performing full cardiac CEST exams using a conventional Gaussian or a tailored pulse for saturation. The tailored pulse resulted in reduced water and MT contrast variation across the myocardium when compared to the conventional pulse. |
||
1118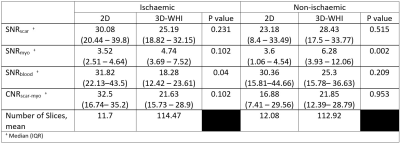 |
23 | Translating 3D Whole Heart LGE to Clinical Practice; Early Feasibility Results in a Tertiary UK Centre
Nikesh Jathanna1, Kevin Strachan1, Bara Erhayiem1, and Shahnaz Jamil-Copley1
1Cardiology and Cardiac Surgery, Nottingham University Hospitals NHS Trust, Nottingham, United Kingdom
Accurately identifying left ventricular fibrosis is paramount for diagnostic, prognostic and procedural reasons. Increasing evidence for the benefit of 3D whole heart imaging in fibrosis identification is emerging. Limitations to integrating 3D whole heart imaging into routine practice include risking suboptimal imaging incumbering diagnostic value. We undertook retrospective quantitative and qualitative analysis of clinically indicated paired 2D and 3D imaging. Quantitative SNRscar and CNRscar-myo analysis was not significantly different and qualitative diagnostic utility was comparable both in patients with ischaemic and non-ischaemic disease. This small study provides evidence for routine usage of 3D whole heart imaging for scar identification.
|
||
1119 |
24 | Imaging cardiac purkinje fibers network using ihMT imaging on a clinical 1.5T scanner
Julie Magat1,2,3, Marylène Delcey4, Solenn Toupin4, Thomas Troalen4, Lucas Soustelle5, Andreea Hertanu5, Olivier Girard5, Guillaume Duhamel5, and Bruno Quesson1,2,3
1Université de Bordeaux, U1045, Bordeaux, France, 2IHU LIRYC, Pessac, France, 3INSERM U1045, Pessac, France, 4Siemens Healthcare SAS, Saint-Denis, France, 5Aix Marseille Univ, CNRS, CRMBM, Marseille, France The Purkinje fiber network (PF) enables electrical impulses throughout the heart and initiate cardiac contraction. To characterize this network in 3D is of high importance to better understand cardiac arrhythmia mechanisms. This preliminary work performed at 1.5T using a high-resolution coil aims to evaluate the inhomogeneous Magnetization Transfer imaging method to visualize PF and differentiate them from the myocardium in a clinical setup. For the first time we obtained an ihMT contrast between a conductive fiber and myocardium at 1.5T on ex vivo sample with an isotropic resolution of 500 µm. |
||
1120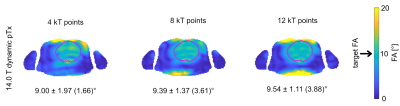 |
25 | Static and dynamic parallel transmission (pTx) for human cardiac MRI at 14.0 T
Bilguun Nurzed1, Thomas Wilhelm Eigentler1,2, Christoph Stefan Aigner3, Sebastian Schmitter3, and Thoralf Niendorf1,4,5
1Berlin Ultrahigh Field Facility (B.U.F.F.), Max-Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany, 2Technische Universität Berlin, Chair of Medical Engineering, Berlin, Germany, 3Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 4MRI.TOOLS GmbH, Berlin, Germany, 5Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max-Delbrück Center for Molecular Medicine, Berlin, Germany
Transmission field inhomogeneities at ultrahigh and extreme field MRI can be offset by using static or dynamic pTx. Responding to the challenges and recognizing the opportunities of cardiac MRI, this abstract examines the feasibility of parallel transmission (pTx) using fractionated dipole (FRD) RF array configurations for static and dynamic B1+ homogenization of the heart at 7.0T and 14.0T. Our results reveal that static pTx provides limited performance at 14.0 T but dynamic pTx enables uniform excitation of the heart at 14.0T. This finding is heartening and provides the technical foundation for explorations into cardiac MRI at 14.0T.
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.