Digital Poster
Deep Learning Image Reconstruction II
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
0944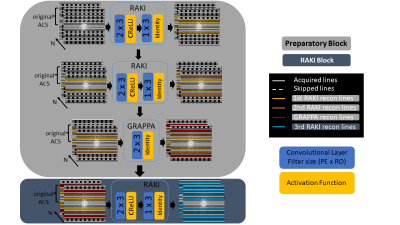 |
64 | Gradual RAKI reconstruction merged with intermittent GRAPPA blurring moderation with limited scan-specific training samples
István Homolya1, Péter Kemenczky1, Peter Dawood2, Zoltán Vidnyánszky1, and Martin Blaimer3
1Brain Imaging Centre, Research Centre for Natural Sciences, Budapest, Hungary, 2Department of Physics, University of Würzburg, Würzburg, Germany, 3Magnetic Resonance and X-Ray Imaging Department, Development Center X-ray Technology EZRT, Fraunhofer Institute for Integrated Circuits IIS, Würzburg, Germany
RAKI is a scan-specific k-space interpolation technique based on deep convolutional networks, which bears superior noise resilience compared to GRAPPA. However, RAKI may introduce severe blurring in image reconstruction due to reduced number of autocalibration signal lines at higher acceleration factors. We propose Gradual RAKI, which exhibits the benefit of mixing RAKI and GRAPPA in a preparatory block for data augmentation purposes prior to a conventional RAKI reconstruction. Data augmentation provides an effective way to create synthetic ACS lines out of 8 original ACS lines at 4-fold acceleration, while valuable features are retained from both RAKI and GRAPPA reconstruction methods.
|
||
0945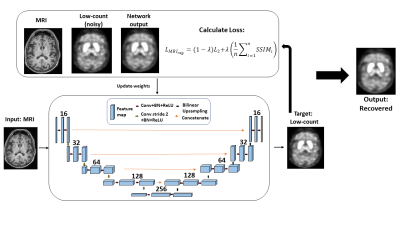 |
65 | MRI Regularization for Low-Count PET Image Recovery using Unsupervised Learning Without the Need of Training Set - A Multi-Tracer PET/MRI Study
Mario Serrano-Sosa1 and Chuan Huang1,2,3
1Biomedical Engineering, Stony Brook University, Stony Brook, NY, United States, 2Radiology, Stony Brook Medicine, Stony Brook, NY, United States, 3Psychiatry, Stony Brook Medicine, Stony Brook, NY, United States
Unsupervised denoising is useful as it allows low-count PET image recovery without the need of paired training data(low-count/full-count). However, current unsupervised denoising models utilize Contrast-to-Noise Ratio as stopping criteria to optimize the image recovery process, which can be improved by considering structural information to maintain the integrity of gross anatomy. In this work, we proposed an MRI structural regularization loss function for low-count PET image recovery using an unsupervised learning model, which does not require paired training sets and demonstrated that the proposed method is superior in both qualitative and quantitative analyses for two radiotracers with very different physiological uptake.
|
||
0946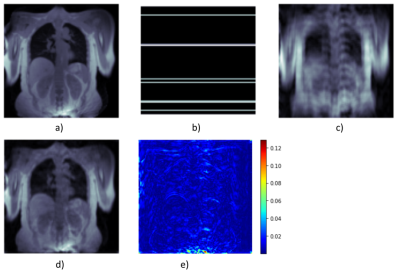 |
66 | Deep Learning based MR reconstruction for accelerated 3D-PREFUL ventilation assessment of post-COVID-19 patients from undersampled MR-images Video Permission Withheld
Maximilian Zubke1,2, Filip Klimeš1,2, Andreas Voskrebenzev1,2, Marcel Gutberlet1,2, Agilo L Kern1,2, Robin A Müller1,2, Arnd J Obert1,2, Frank Wacker1,2, and Jens Vogel-Claussen1,2
1Institute of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany 3D phase-resolved functional lung (3D-PREFUL) proton MRI enables a radiation-free and non-contrast-enhanced ventilation assessment of human lungs. However, generating high-quality images usually requires a long acquisition time. Acceleration can be achieved by undersampling k-space data, but the resulting violation of the Nyquist theorem leads to image artifacts. Deep learning (DL)-based reconstruction approaches are proposed as a solution for this dilemma. Two novel loss functions are introduced to create a deep learning based reconstruction, optimized for lung MRI. The feasibility of ventilation assessment, including ventilation defect identification, from 8x undersampled MR-images of post-COVID-19 patients, reconstructed by a neural network is demonstrated. |
||
0947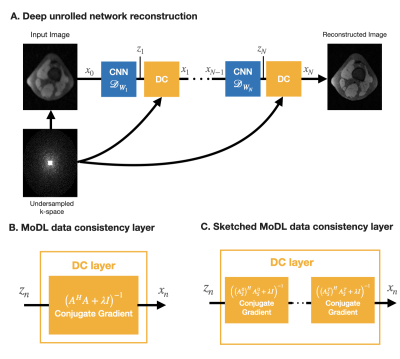 |
67 | Coil-sketched unrolled networks for computationally-efficient deep MRI reconstruction
Julio A Oscanoa1, Batu Ozturkler2, Siddharth S Iyer3,4, Zhitao Li2,4, Christopher M Sandino2, Mert Pilanci2, Daniel B Ennis4, and Shreyas S Vasanawala4
1Department of Bioengineering, Stanford University, Stanford, CA, United States, 2Department of Electrical Engineering, Stanford University, Stanford, CA, United States, 3Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Boston, MA, United States, 4Department of Radiology, Stanford University, Stanford, CA, United States
Deep unrolled networks can outperform conventional compressed sensing reconstruction. However, training unrolled networks has intensive memory and computational requirements, and is limited by GPU-memory constraints. We propose to use our previously developed “coil-sketching” algorithm to lower the computational burden of the data consistency step. Our method reduced memory usage and training time by 18% and 15% respectively with virtually no penalty on reconstruction accuracy when compared to a state-of-the-art unrolled network.
|
||
0948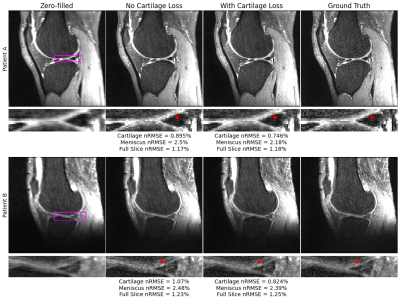 |
68 | A Cartilage-Specific Loss Function Improves Image Reconstruction Performance in Multiple Tissues of Clinical Interest
Aniket Tolpadi1,2, Francesco Calivà1, Misung Han1, Emma Bahroos1, Peder Larson1, Sharmila Majumdar1, and Valentina Pedoia1
1Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 2Bioengineering, University of California, Berkeley, Berkeley, CA, United States
Most MRI image reconstruction algorithms are optimized for full-volume performance rather than specific tissues. Using a KIKI-Net style architecture and multi-component loss function in image space and k-space as baseline, we find a cartilage-specific loss function improves reconstruction performance at R=4 and R=8 in both cartilage and menisci. Thus, it may be possible to improve clinical utility of reconstruction pipelines across tissues of heightened clinical interest using a simple loss function weighting. Furthermore, full-volume standard reconstruction metrics worsened at R=4 and R=8 while tissue-specific metrics improved, calling into question whether these metrics are best for assessing reconstruction pipeline clinical utility.
|
||
0949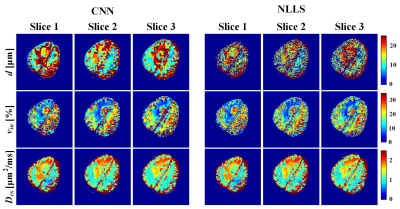 |
69 | IMPULSED model-based brain tumor microstructural parameter estimation with deep neural network Video Not Available
Jian Wu1, Taishan Kang2, Xinran Chen1, Lina Xu1, Jianzhong Lin2, Zhigang Wu3, Tianhe Yang2, Congbo Cai1, and Shuhui Cai1
1Xiamen University, Xiamen, China, 2Zhongshan Hospital Afflicated to Xiamen University, Xiamen, China, 3MSC Clinical & Technical Solutions, Philips Healthcare, Shenzhen, China
This study assesses the feasibility of training a convolutional neural network (CNN) for IMPULSED (imaging microstructural parameters using limited spectrally edited diffusion) model fitting to diffusion-weighted (DW) data and evaluates its performance on a brain tumor (poorly differentiated adenocarcinoma) patient data directly acquired from clinical MR scanner. Comparisons were made with the results calculated from the non-linear least squares (NLLS) algorithm. More accurate and robust results were obtained by our CNN method, with processing speed several orders of magnitude faster than the reference method (from 5 min to 1 s).
|
||
0950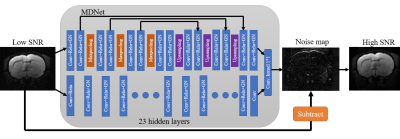 |
70 | New Denoising Neural Network for Diffusion Tensor Imaging Signal-to-Noise Ratio Enhancement Video Not Available
Po-Ting Chen1, Tzu-Yi Wang1, and Jyh-Horng Chen1
1National Taiwan University, Taipei, Taiwan
本研究提出了一種新的神經網絡模型來提高 DTI 圖像的信噪比。從實驗結果來看,我們成功地將圖像的 SNR 提高了 3 倍,相當於減少了 9 倍的掃描時間。這將改善因信噪比不足而導致的 DTI 分析困難。
|
||
0951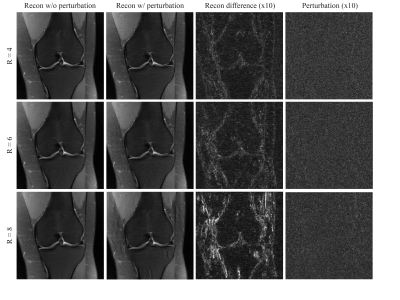 |
71 | Assessment of Instabilities of Conventional and Deep Learning Multi-Coil MRI Reconstruction at Multiple Acceleration Factors
Ruoxun Zi1, Patricia Johnson1,2, and Florian Knoll3
1Center for Biomedical Imaging, Department of Radiology, NYU School of Medicine, New York, NY, United States, 2Center for Advanced Imaging Innovation and Research (CAI2R), NYU School of Medicine, New York, NY, United States, 3Department Artificial Intelligence in Biomedical Engineering, FAU Erlangen-Nuremberg, Erlangen, Germany
Although deep learning has received much attention for accelerated MRI reconstruction, it shows instabilities to certain tiny perturbations resulting in substantial artifacts. There has been limited work comparing the stability of DL reconstruction with conventional reconstruction methods such as parallel imaging and compressed sensing. In this work, we investigate the instabilities of conventional methods and the Variational Network (VN) with different accelerations. Our results suggest that CG-SENSE with an optional regularization is also impacted by perturbations but shows less artifacts than the VN. Each reconstruction method becomes more vulnerable with higher acceleration and VN shows severe artifacts with 8-fold acceleration.
|
||
0952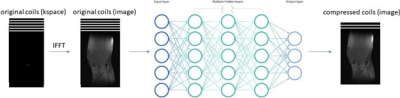 |
72 | Improved Neural Network-Based Coil Compression
Elizabeth K. Cole1,2, Qingxi Meng1,2, Anishka Raina3, John M. Pauly2, and Shreyas S. Vasanawala4
1Equal Contribution, Stanford, CA, United States, 2Electrical Engineering, Stanford University, Stanford, CA, United States, 3The Harker School, San Jose, CA, United States, 4Rad/Pediatric Radiology, Stanford University, Stanford, CA, United States
Coil compression is performed in magnetic resonance imaging (MRI) to enable smaller datasets and faster computation time. However, the traditional coil compression process is lengthy and lossy. In this work, we proposed a novel neural network-based coil compression method to achieve higher reconstruction accuracy and faster coil compression. Our method consistently achieved up to 1.5x lower NRMSE compared to SVD and GCC on the fastMRI knee dataset. The computational requirements of our method are practical, and inference runs 10 times faster than the traditional methods.
|
||
0953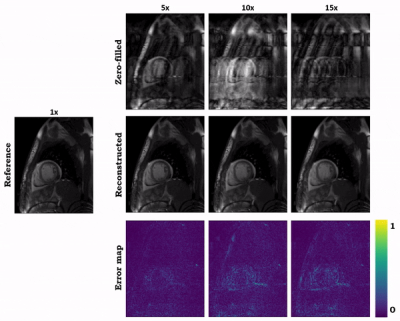 |
73 | Hybrid image and k-space deep learning reconstruction exploiting spatio-temporal redundancies for 2D cardiac CINE
Siying Xu1, Patrick Krumm2, Andreas Lingg2, Haikun Qi3, Kerstin Hammernik4,5, and Thomas Küstner1
1Medical Image And Data Analysis (MIDAS.lab), Department of Interventional and Diagnostic Radiology, University Hospital of Tuebingen, Tuebingen, Germany, 2Department of Radiology, University Hospital of Tuebingen, Tuebingen, Germany, 3School of Biomedical Engineering, ShanghaiTech University, Shanghai, China, 4Lab for AI in Medicine, Technical University of Munich, Munich, Germany, 5Department of Computing, Imperial College London, London, United Kingdom
Cardiac CINE MR imaging allows for accurate and reproducible measurement of cardiac function but requires long scanning times. Parallel imaging and compressed sensing (CS) have reduced the acquisition time, but the possible acceleration remained limited. Deep learning-based MR image reconstructions can further increase the acceleration rates with improved image quality. In this work, we propose a novel network for retrospectively undersampled 2D cardiac CINE that a) operates in image and k-space domain with interleaved architectures and b) utilizes spatio-temporal filters for multi-coil dynamic data. The proposed network outperforms CS and some other neural networks in both qualitative and quantitative evaluations.
|
||
0954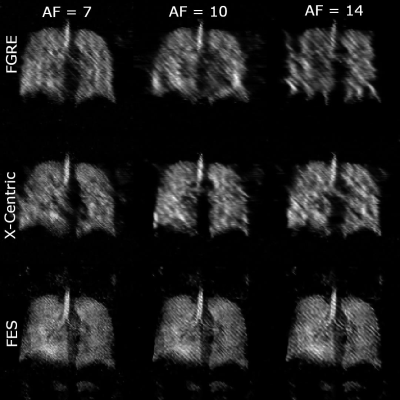 |
74 | Improvements of Image Quality of 1H and 129Xe MRI by Using an Advanced Acquisition and Reconstruction Method Coupled with Deep Learning
Samuel Perron1, Matthew S. Fox1,2, and Alexei Ouriadov1,2,3
1Physics and Astronomy, The University of Western Ontario, London, ON, Canada, 2Lawson Health Research Institute, London, ON, Canada, 3School of Biomedical Engineering, The University of Western Ontario, London, ON, Canada Accelerated MRI has the potential to significantly improve image quality without increasing costs, especially for low field strengths. A series of undersampled images are averaged for every unique permutation, and their SNR dependency is fitted to the Stretched-Exponential-Model. The proposed method was implemented in proactively undersampled phantom images at low field (0.074T) and in retroactively undersampled human lung images at high field (3T) using the FGRE pulse sequence; in all cases, SNR was significantly improved within the same scan duration compared to a fully-sampled image. Reconstruction artefacts were minimized or completely removed using a convolutional neural network. |
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.