Digital Poster
Brain Tumors II
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
2742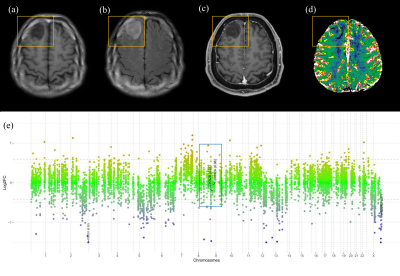 |
10 | Qualitative and Quantitative Imaging Phenotypes can Predict CDKN2A/B Homozygous Deletion Status in IDH-mutant Astrocytomas
Yae Won Park1, Ki Sung Park2, Sung Soo Ahn1, Inho Park1, and Se Hoon Kim1
1Yonsei University College of Medicine, Seoul, Korea, Republic of, 2Pohang University of Science and Technology, Seoul, Korea, Republic of
CDKN2A/B homozygous deletion is a key molecular marker in the 2021 WHO classification of IDH-mutant astrocytomas; presence of CDKN2A/B homozygous deletion results in a grade of 4. On multivariable analysis, infiltrative pattern, larger tumor volume, and higher 95th percentile of nCBV were independent predictors of CDKN2A/B homozygous deletion. The infiltrative pattern and larger volume may be reflected from the increased tumor cell proliferation, whereas the higher 95th percentile of nCBV may reflect the increased angiogenesis due to CDKN2A/B homozygous deletion.
|
||
2743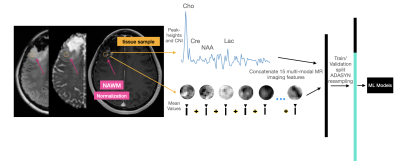 |
11 | Tissue-level probabilistic mapping of treatment-induced effects in recurrent glioblastoma
Jacob Ellison1,2,3, Nate Tran1,2,3, Julia Cluceru1,2, Joanna Phillips4,5, Anny Shai5, Devika Nair1, Annette Molinaro5, Valentina Pedoia1,2,3, Yan Li1,2,3, Javier Villanueva-Meyer1, Mitchel Berger5, Shawn Hervey-Jumper5, Manish Aghi5, Susan Chang5, and Janine Lupo1,2,3
1Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 2Center for Intelligent Imaging, UCSF, San Francisco, CA, United States, 3Graduate Group in Bioengineering, UCSF - UC Berkeley, San Francisco, CA, United States, 4Brain Tumor Research Center, UCSF, San Fransisco, CA, United States, 5Neurological Surgery, UCSF, San Francisco, CA, United States
Treatment-induced effects can mimic tumor recurrence and pose a challenge to accurately assessing treatment response. We aim to provide a machine learning framework and identify important imaging features for discriminating treatment-induced injury from recurrent glioblastoma at biopsy level resolution. Our best model performs with a mean AUC of .77+/-0.11 across 4 fold cross-validation of 108 tissue samples. rCBV, choline-to-NAA index (CNI), and normalized lipid levels were the top three most import features in distinguishing treatment effects from recurrent tumor.
|
||
2744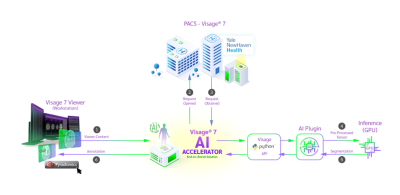 |
12 | Clinical Implementation of Novel PACS-based Deep Learning Glioma Segmentation Algorithm
Sara Merkaj1, Khaled Bousabarah2, Lin MingDe1, Andrej Pala3, Gabriel Cassinelli Petersen1, Leon Jekel1, Ryan Bahar1, Niklas Tillmanns4, Ajay Malhotra1, Malte Westerhoff2, and Mariam Aboian1
1Yale School of Medicine, New Haven, CT, United States, 2Visage Imaging, Berlin, Germany, 3Ulm University, Ulm, Germany, 4University of Düsseldorf, Düsseldorf, Germany
Tumor segmentation is a laborious process, which impedes the progress of data production for development of classification/prediction algorithms. We present a novel PACS-based workflow for deep learning-based auto-segmentation of gliomas that allows generation of annotated images during clinical workflow. We developed a U-Net auto-segmentation algorithm natively imbedded in PACS and trained on BraTS dataset. Subsequent retraining on tertiary hospital dataset was performed and generation of new segmentations, allowing labeling of 440 gliomas in a three-months period. This novel approach for annotated data generation allows real-time building of large, labeled datasets by experts in the field.
|
||
2745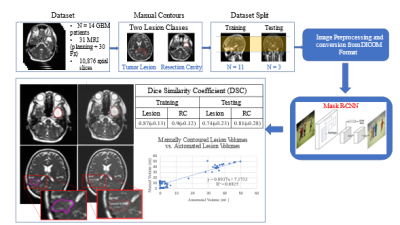 |
13 | A Deep Learning Approach for Automated Volume Delineation on Daily MRI Scans in Glioblastoma Patients
Adrian Lazaro Breto1, Kaylie Cullison2, Kolton Jones3, Olmo Zavala-Romero4, John C Ford1, Eric A Mellon1, and Radka Stoyanova1
1Radiation Oncology, University of Miami Sylvester Comprehensive Cancer Center, Miami, FL, United States, 2University of Miami Miller School of Medicine, Miami, FL, United States, 3West Physics, Atlanta, GA, United States, 4Department of Earth, Ocean, and Atmospheric Sciences, Florida State University, Tallahassee, FL, United States Identifying early progressors following treatment for Glioblastoma (GBM) is paramount in GBM management. MRI-RT platforms provide opportunity for daily MRI of patients. We identified early changes in tumor volume typically starting week 3 or 4 of treatment. We hypothesize tumor volume kinetics are associated with outcome and allow for adapting treatment. We develop a deep learning solution for automatic volume delineation on daily scans, allowing real time monitoring of tumor changes and reducing time burden of segmentation. We obtained DSC for tumor lesion and resection cavity on training and test datasets (mean±standard deviation) 0.87±0.128 and 0.9±0.122; 0.74±0.233 and 0.8±0.277, respectively. |
||
2746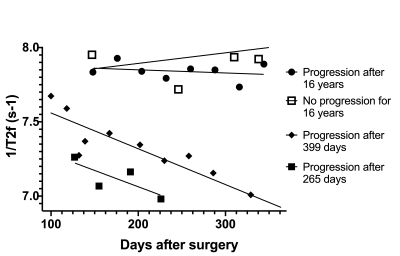 |
14 | T2 Relaxometry in the Prediction of Progression-Free Survival in Patients with Primary Glioblastoma: Whole Brain and Deep Learning Approach
Aaron Rulseh1 and Josef Vymazal1
1Dept. of Radiology, Na Homolce Hospital, Prague, Czech Republic
Glioblastoma (GBM) is the most common and aggressive primary brain tumor. Any methods that may improve confirmation or early detection of progression are highly desirable. T2 relaxometry shows great promise in the monitoring of GBM patients following complex therapy. We found WB metrics, such as median 1/T2, correlated with PFS and were able to distinguish progression from pseudo-progression. A deep neural network trained on automatically segmented 1/T2 data achieved an F1 accuracy of 85% in classifying progression with a threshold of 18 months.
|
||
2747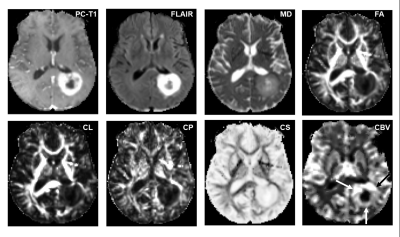 |
15 | ASSESSMENT OF TREATMENT RESPONSE TO DC VACCINE IN RECURRENT GBM PATIENTS USING MULTIPARAMETRIC MRI PREDICTION MODEL
Laiz Laura de Godoy1, Sumei Wang1, Shadi Asadollahi1, MacLean P. Nasrallah1, Donald M. O’Rourke1, Steven Brem1, Arati Desai1, Laurie Loevner1, Suyash Mohan1, and Sanjeev Chawla1
1Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States
The highly aggressive nature of glioblastoma (GBM) leads to a dismal prognosis, and alternative therapies are being sought. Immunotherapy, such as the Dendritic Cell (DC) vaccine, is currently being evaluated for recurrent GBMs in clinical trials. Follow-up imaging after this immunotherapeutic approach often mimics disease progression on conventional images, making standard MRI evaluation challenging. Our findings indicate that our previously established multiparametric MRI-based prediction model has the capacity to accurately assess response to DC vaccine in recurrent GBM patients, objectively characterizing as either TP or PsP GBM patients, avoiding interruptions in satisfactory treatments, and preventing invasive procedures in PsP cases.
|
||
2748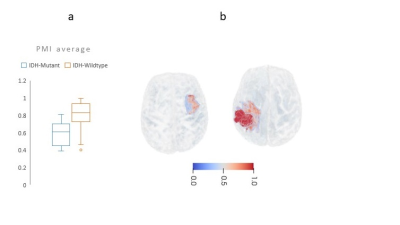 |
16 | AI-driven markers of IDH1 mutational status using microstructure-based characterization of peritumoral region in gliomas
Zahra Riahi Samani1, Drew Parker1, Steven Brem2, and Ragini Verma1
1DiCIPHR (Diffusion and Connectomics in Precision Healthcare Research) Lab, University of Pennsylvania, Philadelphia, PA, United States, 2Department of Radiology, Department of Neurosurgery, University of Pennsylvania, Philadelphia, PA, United States
We developed a novel, microstructure-based, voxel-wise map of the peritumoral region in glioma brain tumors using DTI-based free water volume fraction map and deep-learning. This novel map captures the infiltrative heterogeneity of peritumoral region and can differentiate between gliomas with distinct IDH1 mutation status (IDH-mutant vs. IDH-wildtype). Thus, this new derived map that incorporates microstructure information can be used as a new diffusion based radiomic feature for various oncological investigations involving mutation status.
|
||
2749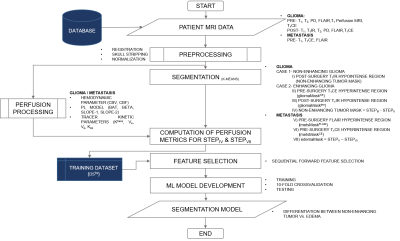 |
17 | Machine Learning based Differentiation of Non-Enhancing Tumor from Vasogenic Edema in patients with Low Grade Gliomas using DCE-MRI
Virendra Kumar Yadav1, Rakesh Kumar Gupta2, Sumeet Agarwal3, and Anup Singh1,4
1Center for Biomedical Engineering, Indian Institute of Technology, Delhi, India, 2Fortis Memorial Research Institute, Gurugram, India, 3Electrical Engineering, Indian Institute of Technology, Delhi, India, 4Biomedical Engineering, All India Institute of Medical Sciences, Delhi, India Precise safe surgical resection and precisely directed radiation are important in clinical practice for low-grade gliomas (LGGs) patients in order to minimize the neurological deficit and radiation toxicity, respectively. Clinicians find difficulty in defining the border between the non-enhancing tumor and edema components. In this study, machine learning-based models were developed in order to distinguish non-enhancing tumors from vasogenic edema using quantitative perfusion parameters obtained using dynamic-contrast-enhanced MRI. The proposed approach may help in assisting radiologists, by defining precise tumor boundaries and hence, results in improving patients’ quality of life and overall survival. |
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.