Digital Poster
Acquisition & Analysis Methods for fMRI
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
1096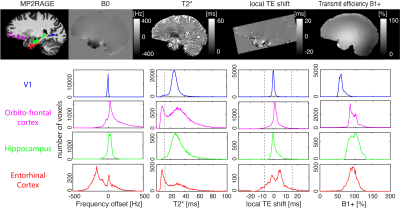 |
1 | Why is 7T fMRI in the entorhinal cortex so difficult and what can we do about it?
Nadine N Graedel1, Oliver Warrington1, Barbara Dymerska1, Vahid Malekian1, Oliver Josephs1, Peter Kok1, and Martina F Callaghan1
1Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom
Functional MRI in the entorhinal cortex is very challenging due to large and geometrically complex B0 field inhomogeneity. As a result, a quarter of voxels are lost due to T2*-driven dephasing and/or because the echo is pushed outside of the acquisition window. Additionally, off-resonant voxels suffer from poor excitation efficiency with frequency-selective binomial pulse excitation, which are preferred for fat suppression for SAR and time efficiency. We empirically demonstrate substantial signal, and consequently tSNR, gains in off-resonance regions by reducing binomial pulse order (from 1-3-3-1 to 1-2-1 or 1-1) that match numerical predictions.
|
||
1097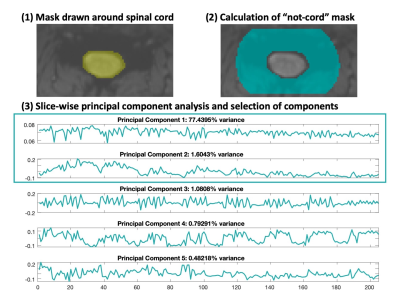 |
2 | SpinalCompCor: PCA-based denoising for spinal cord fMRI
Kimberly J. Hemmerling1,2, Mark A. Hoggarth2, Todd Parrish3, Robert L. Barry4,5,6, and Molly G. Bright1,2
1Biomedical Engineering, Northwestern University, Evanston, IL, United States, 2Physical Therapy & Human Movement Sciences, Northwestern University, Chicago, IL, United States, 3Department of Radiology, Northwestern University, Chicago, IL, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 5Department of Radiology, Harvard Medical School, Boston, MA, United States, 6Harvard–Massachusetts Institute of Technology Health Sciences & Technology, Cambridge, MA, United States
SpinalCompCor is a denoising technique in which principal component (PC) analysis is performed in a region outside of the spinal cord to define nuisance regressors. Temporal SNR was greater when PC regressors were included in the general linear model, compared to when they were regressed out of the data prior to motion correction. In comparing models that vary the number of PC regressors, models with multiple PCs generally performed better than those with single PCs. SpinalCompCor is a promising technique for spinal cord fMRI denoising.
|
||
1098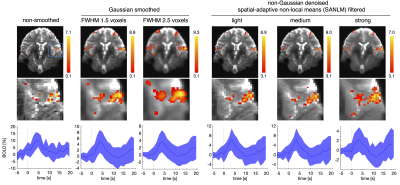 |
3 | Non-Local Means Denoising of 7T Functional MR Images: Enhancing Spatial Accuracy of Fine-Grained Task-Specific Neurosignatures?
Igor Fabian Tellez Ceja1, Thomas Gladytz1, Ludger Benedikt Starke1, Karsten Tabelow2, Thoralf Niendorf1,3, and Henning Matthias Reimann1
1Berlin Ultrahigh Field Facility (B.U.F.F.), Max Delbrück Center for Molecular Medicine, Berlin, Germany, 2Weierstrass Institute for Applied Analysis and Stochastics, Berlin, Germany, 3Experimental and Clinical Research Center (ECRC), a joint cooperation between the Charité Medical Faculty and the Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Berlin, Germany
Superior spatial fidelity of fMRI at ultrahigh magnetic field strengths (≥7T) in principle allows for resolving fine grained task-specific fMRI neurosignatures. Yet, spatial details of BOLD clusters are conventionally blurred by Gaussian smoothing. To preserve spatial details, spatial-adaptive non-local means (SANLM) denoising has been introduced in fMRI as an alternative to Gaussian smoothing. Here, we evaluate SANLM denoising at 7T. SANLM removes noise in homogeneous areas while maintaining edges. It prevents the spread of BOLD patterns between adjacent brain regions, but should be employed with caution, as its spatial detail is partially driven by the underlying anatomy.
|
||
1099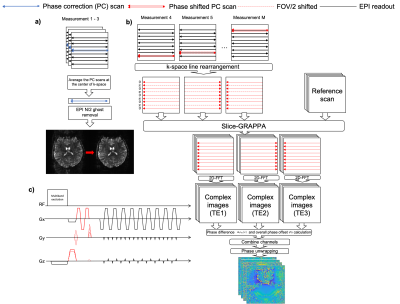 |
4 | Field-Mapping-Embedded EPI for Geometric Distortion Correction
Didi Chi1, Yasmin Blunck1,2, Rebecca Glarin2, Catherine E. Davey1,2, Daniel Staeb3, and Leigh A. Johnston1,2
1Department of Biomedical Engineering, University of Melbourne, Parkville, Australia, 2Melbourne Brain Centre Imaging Unit, University of Melbourne, Parkville, Australia, 3MR Research Collaborations, Siemens Healthcare Pty Ltd, Melbourne, Australia
A Field-Mapping-Embedded (FME) EPI sequence is proposed in which the map is acquired during EPI acquisition without increasing scan time, using phase-encoded phase correction navigators. Results from in vivo experiment demonstrate accurate measurement and robust geometric distortion that performs favourably in comparison with existing techniques that require additional scans.
|
||
1100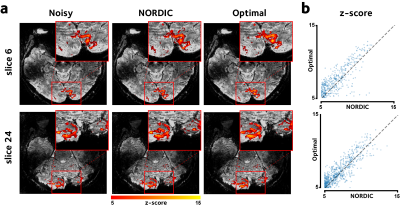 |
5 | Optimal Singular-Value Shrinkage for fMRI Denoising
Mo Shahdloo1 and Mark Chiew1
1Wellcome Centre for Integrative Neuroimaging (WIN), FMRIB, University of Oxford, Oxford, United Kingdom
Singular-value truncation techniques have shown promise for reducing thermal noise in fMRI, where singular-values below a certain threshold are assumed to be noise and are discarded. However, this approach could lead to suboptimal signal recovery, since the remaining singular-values could still have variance contributed by noise. Here instead we propose to use a theoretically MSE-optimal function to shrink the remaining singular-values. The proposed method is evaluated using simulations and high-resolution in-vivo human brain data, and is shown to improve signal-to-noise ratio and functional statistics while leaving the spatial precision intact.
|
||
1101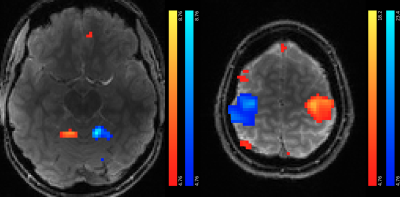 |
6 | T-Hex spirals for whole-brain fMRI at 5 frames per second: Image quality and BOLD sensitivity
Samuel Bianchi1, Maria Engel1, Jakob Heinzle2, Stefan Frässle2, Klaas E. Stephan2,3, and Klaas P. Pruessmann1
1Institute for Biomedical Engineering, ETH Zurich & University of Zurich, Zürich, Switzerland, 2Translational Neuromodeling Unit, University of Zurich & ETH Zurich, Zürich, Switzerland, 3Max Planck Institute for Metabolism Research, Cologne, Germany
fMRI is one of the driving technologies of neuroscience. During the past years, acquisition speeds below one second per whole-brain volume have been achieved. The recently proposed 3D T-Hex Spiral trajectories provide a powerful approach to enable even faster fMRI. Here, we demonstrate the feasibility of fMRI at a sampling rate of 5Hz with these trajectories at 7T. We acquired whole-brain images of one subject performing a visuo-motor task in the scanner. Our results indicate high BOLD sensitivity and fidelity of voxel timeseries. In total, we analyzed 9000 volumes obtained during 30min of scanning.
|
||
1102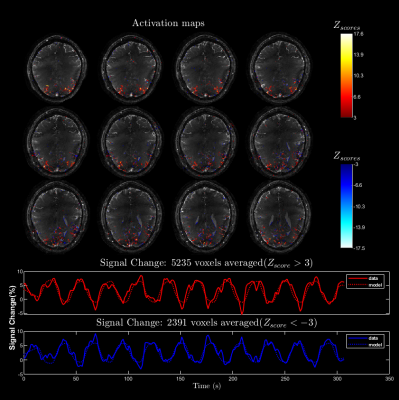 |
7 | Accelerated 3D stack-of-spiral bSSFP for functional imaging at 9.4T: Pilot study
Praveen Iyyappan Valsala1, Philipp Ehses2, Marten Veldmann2, and Klaus Scheffler1,3
1Magnetic Resonance Center, Max-Planck Institute for Biological Cybernetics, Tübingen, Germany, 2German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 3Department of Biomedical Magnetic Resonance, Eberhard Karls University Tübingen, Tübingen, Germany We investigate the possibility of using longer multi-shot spiral readouts to accelerate balanced steady-state free precession (bSSFP) at ultra-high field (UHF) for functional imaging. The reconstruction is performed with the full signal model accounting for spatial B0 inhomogeneity and gradient imperfections. We present the activation maps from flickering checkerboard stimulus experiment and discuss challenges of spiral bSSFP at UHF. |
||
1103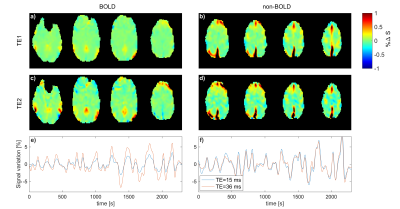 |
8 | Multi-Echo MR-Encephalography using Spherical Stack of Spirals Trajectories
Antonia Barghoorn1, Bruno Riemenschneider2, Wenchao Yang1, and Jürgen Hennig1
1Department of Radiology, Medical Physics, University Medical Center Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 2Department of Artificial Intelligence in Biomedical Engineering, Friedrich-Alexander University Erlangen-Nürnberg, Erlangen, Germany
MREG allows very fast measurement of physiological signal changes at 100 ms acquisition time per volume. In order to investigate BOLD and non-BOLD contributions to the observed signal variations we have implemented an interleaved measurement scheme based on signal readout with two different echo times (15 and 36 ms). The interleaved implementation with alternating echo times increases the acquisition time to 200 ms, which still allows to investigate BOLD vs. non-BOLD signal changes at frequencies up to ±2.5 Hz. Preliminary measurements demonstrate the feasibility of ME-MREG in detecting both neuronal activations and resting-state functional connectivities.
|
||
1104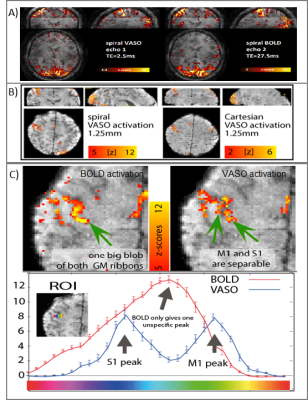 |
9 | VASO fMRI USING 2D-SMS SPIRAL READOUTS
Denizhan Kurban1, Renzo Huber1, Gilad Liberman2, Dimo Ivanov1, and Benedikt A Poser1
1Maastricht University, Maastricht, Netherlands, 2Athinoula A. Martinos Center for Biomedical Imaging, MGH, Boston, MA, United States
High-resolution blood volume-sensitive fMRI VASO can capture functional signal changes with high localization specivity compared to conventional BOLD sequences. However, it suffers from reduced sampling efficiencies and lower detection sensitivity. Here, we overcome these limitations by combining VASO contrasts with the high efficiency of multi-echo enabled spiral k-space sampling and CAIPI-optimized SMS acceleration. We find that spiral sampling enables faster VASO acquisitions than possible with Cartesian EPI sampling. For high spatial resolutions (1.25mm), we confirm that the proposed method is insensitive to large draining veins while having more stable fMRI signals (higher tSNR).
|
||
1105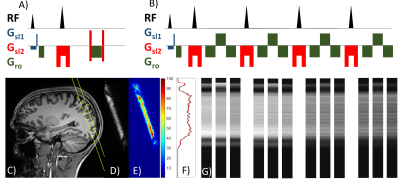 |
10 | Simultaneous acquisition of GRE- and SE-type resting-state fMRI signals with GRASE-based line-scanning in the human brain
Sangcheon Choi1,2, Xin Yu3, Klaus Scheffler1,4, and Kai Herz1,4
1High-Field Magnetic Resonance, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Graduate Training Centre of Neuroscience, University of Tuebingen, Tuebingen, Germany, 3Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Harvard Medical School, Massachusetts General Hospital, Charlestown, MA, United States, 4Biomedical Magnetic Resonance, University of Tuebingen, Tuebingen, Germany
GRE- or SE-based line-scanning fMRI methods can characterize dynamic fMRI signals with high spatial and temporal resolution. Here, we present a combination of both using a 1D GRASE sequence with perpendicular excitation and refocusing slices. We applied the sequence in-vivo at 3T and analyzed slice pofiles and tSNR.
|
||
1106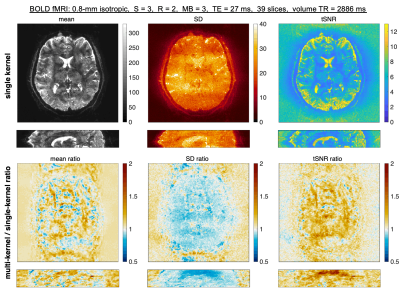 |
11 | Improved high-resolution fMRI image quality with simultaneous multislice VFA-FLEET using a novel multi-kernel slice-GRAPPA algorithm Video Not Available
Avery JL Berman1,2, Kawin Setsompop3,4, Thomas Witzel5, William A Grissom6,7, and Jonathan R Polimeni1,2,8
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Department of Radiology, Stanford University, Palo Alto, CA, United States, 4Department of Electrical Engineering, Stanford University, Palo Alto, CA, United States, 5Q Bio, Inc., San Carlos, CA, United States, 6Vanderbilt University Institute of Imaging Science, Vanderbilt University, Nashville, TN, United States, 7Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 8Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States We propose a novel slice-GRAPPA reconstruction algorithm, termed multi-kernel slice-GRAPPA (mks-GRAPPA), to tackle the challenge of reconstructing high spatial resolution segmented multi-shot EPI data for fMRI. This is particularly relevant for the recently proposed Variable-Flip-Angle “FLEET” pulse sequence. For a segmentation factor, S, by training 2×S slice-GRAPPA kernels, rather than one, we demonstrate significant improvements in image quality metrics under a wide range of protocols. The multitude of kernels account for static signal discontinuities within and across segments in multi-shot EPI. In the SNR starved regime of high-res fMRI, mks-GRAPPA allows us to recover a significant portion of lost tSNR. |
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.