Digital Poster
Novel Image Reconstruction Techniques I
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
2339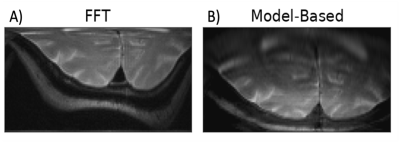 |
75 | Iterative Model-Based Image Reconstruction of RF gradient-based MRI
Taylor Froelich1 and Michael Garwood1
1Center for Magnetic Resonance Research and Department of Radiology, University of Minnesota, Minneapolis, MN, United States
When reconstructing with traditional Fourier-based techniques, image distortions arising from nonlinear B1 and B0 inhomogeneity can plague radiofrequency-based (RF) imaging methods that rely on B1 gradients for spatial encoding. In this work, we propose a new framework for reconstructing multi-dimensional RF gradient-based images leveraging an iterative approach to solve a regularized inverse problem. The proposed methodology employs a full Bloch simulation to reconstruct an undistorted image after determination of the forward operator and measured receive coil sensitivities.
|
||
2340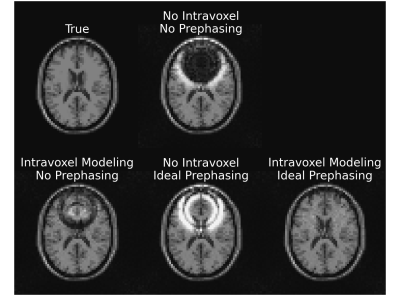 |
76 | Intravoxel B0 Corrected Image Reconstruction with RF Prephasing
Steven T. Whitaker1, Jon-Fredrik Nielsen2, and Jeffrey A. Fessler1
1Electrical Engineering and Computer Science, University of Michigan, Ann Arbor, MI, United States, 2Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States
Regions with large intravoxel B0 gradients result in a wide spread of off-resonance frequencies within each voxel, causing spins within a voxel to dephase with respect to each other. Using model-based reconstruction to account for this dephasing can help alleviate artifacts from this signal loss, but success is limited in areas of extreme dephasing. We propose a model-based reconstruction method that includes RF prephasing to help mitigate the effects of extreme dephasing. We demonstrate that the proposed approach successfully recovers signal in areas of extreme dephasing and results in lower reconstruction error than model-based reconstruction without RF prephasing.
|
||
2341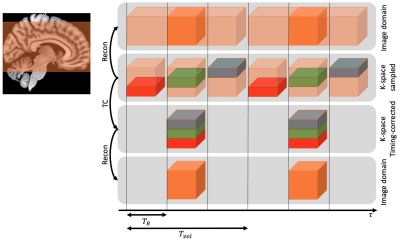 |
77 | Reconstruction of 3D EPI timeseries including a correction of different acquisition times
Samuel Bianchi1, Jakob Heinzle2, Maria Engel1, Lars Kasper1,2, and Klaas P. Pruessmann1
1Institute for Biomedical Engineering, ETH Zurich & University of Zurich, Zürich, Switzerland, 2Translational Neuromodeling Unit, University of Zurich & ETH Zurich, Zürich, Switzerland
Standard fMRI acquires volumes as stacks of slices with 2D sequences. Acquisition time differences within a volume can be corrected post hoc using a sinc interpolation in image space (“slice timing correction”). In 3D sequences, timing correction is not possible in image space. Here, we verify an approach that rests on a sinc interpolation of timeseries in k-space before image reconstruction. Particularly, we investigate the artifacts which get removed by timing correction and quantify effects on the power spectra of timeseries in image space for 3D EPI.
|
||
2342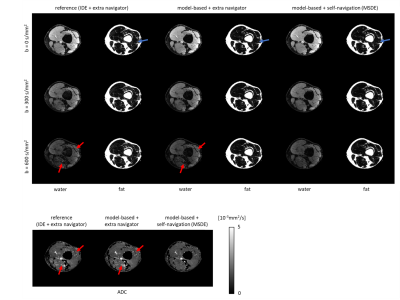 |
78 | Model-based self-navigated water/fat decomposition for segmented diffusion-weighted EPI
Yiming Dong1, Malte Riedel2, Kirsten Koolstra3, Matthias J.P. van Osch1, and Peter Börnert1,4
1C.J. Gorter Center for High Field MRI, Department of Radiology, LUMC, Leiden, Netherlands, 2University and ETH Zurich, Zurich, Switzerland, 3Division of Image Processing, Department of Radiology, LUMC, Leiden, Netherlands, 4Philips Research, Hamburg, Germany
Multi-shot EPI readout-approaches provide high spatial resolution at reduced geometric distortions and improved SNR in diffusion weighted imaging (DWI). As a specific challenge, multi-shot acquisition data require corrections for motion-induced, shot-specific phase errors, e.g. using additional navigator signals or appropriate self-navigation. Furthermore, proper fat-suppression is challenging in DWI, especially at B0 critical regions, making the use of chemical-shift encoding interesting. Therefore, an iterative, model-based reconstruction algorithm with self-navigation and water/fat decomposition, is proposed in this work. In-vivo examples in the leg and head-neck regions demonstrate improved water/fat separation as compared to acquisition-navigator approaches, while measurement times can be shortened.
|
||
2343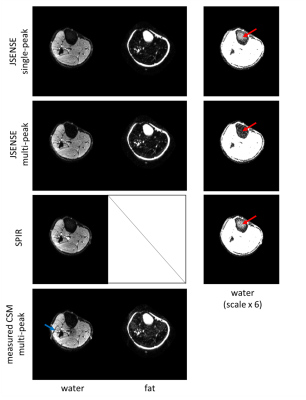 |
79 | SENSE-based multipeak water/fat separation for diffusion-weighted imaging using self-navigated interleaved EPI
Yiming Dong1, Kirsten Koolstra2, Malte Riedel3, Matthias J.P. van Osch1, and Peter Börnert1,4
1C.J. Gorter Center for High Field MRI, Department of Radiology, LUMC, Leiden, Netherlands, 2Division of Image Processing, Department of Radiology, LUMC, Leiden, Netherlands, 3University and ETH Zurich, Zurich, Switzerland, 4Philips Research, Hamburg, Germany
The presence of fat signals is one challenge for diffusion-weighted EPI, especially when considering the multi-peak spectrum nature of fat. In this work, we propose an improved SENSE-based water/fat separation algorithm to suppress multi-peak fat signals and apply this specifically to diffusion-weighted multi-shot EPI. The motion-induced shot-to-shot phase variations, an inevitable challenge in multi-shot DWI, are incorporated into the signal model using either a self-navigation or an extra-navigated method. The results show that the proposed SENSE-based algorithm yields good water/fat separation for non-diffusion and diffusion data with a multi-peak fat spectrum model.
|
||
2344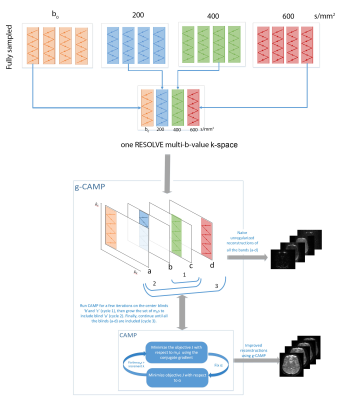 |
80 | g-CAMP reconstructs multiple b-value diffusion weighted images from a single RESOLVE k-space
Nahla M H Elsaid1, Hemant D Tagare1,2, and Gigi Galiana1
1Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, CT, United States, 2Department of Biomedical Engineering, Yale University, New Haven, CT, United States
This work demonstrates the feasibility of reconstructing multiple b-value diffusion-weighted images (DWI) from a single RESOLVE k-space, where each blind was acquired with a different b-value. This multi b-value reconstruction uses the growing Constrained Alternating Minimization for Parameter mapping (g-CAMP) reconstruction method previously presented in ISMRM 2021. This can allow undersampling in the diffusion weighting dimension that is compatible with common undersampling schemes such as GRAPPA and multi-band EPI.
|
||
2345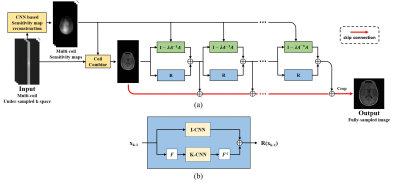 |
81 | Multi-domain Neumann Network with Sensitivity maps for Parallel MRI Reconstruction
Junhyeok Lee1, Junghwa Kang1, Se-hong Oh1, and Dong Hye Ye2
1Biomedical Engineering, Hankuk University of Foreign Studies, Yongin-si, Gyeonggi-do, Korea, Republic of, 2Department of Electrical and Computer Engineering, Marquette University, Milwaukee, WI, United States
We performed parallel MRI reconstruction from under-sampled k-space data using the Multi-Domain Neumann Network with Sensitivity Maps. The Neumann network solves the inverse problem with recursive neural networks taking account into the forward model. We adapt the Neumann network with the coil sensitivity estimation and k-space data regurlaization to take account into MR physical models. Our proposed method shows uppressed image artifacts and enhanced spatial resolution compared with GRAPPA, U-Net and Neumann network.
|
||
2346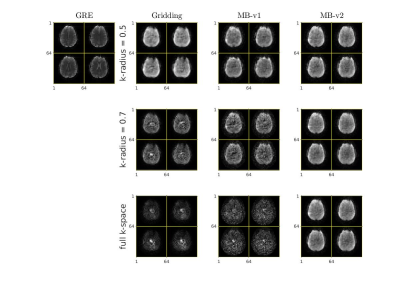 |
82 | Model-based Image Reconstruction in Looping-star MRI
Haowei Xiang1, Jeffrey A Fessler1, and Douglas C Noll2
1Electrical Engineering and Computer Science, University of Michigan, Ann Arbor, Ann Arbor, MI, United States, 2Biomedical Engineering, University of Michigan, Ann Arbor, Ann Arbor, MI, United States
Looping star is a silent MRI pulse sequence that can be used for quantitative susceptibility mapping (QSM), T2*-weighted imaging and fMRI. The conventional reconstruction approach for looping star MRI that filters out some the k-space data does not fully model the overlapping echoes, and remove potentially useful signals. This work proposes a model-based reconstruction method that can theoretically resolve the overlapping echos and improve the SNR without increasing the scan time.
|
||
2347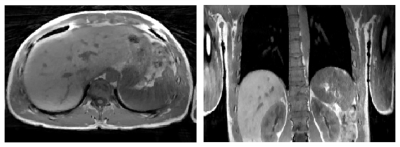 |
83 | Eliminating limits of spatiotemporal resolution in radial stack-of-stars imaging using FID navigators and single-readout binning
I. T. Maatman1, S. Ypma1, M. Kachelrieß2, Y. Berker2, K. T. Block3, E. Van der Bijl1, J. J. Hermans1, M. C. Maas1, and T. W. J. Scheenen1
1Radboud University Medical Centre, Nijmegen, Netherlands, 2German Cancer Research Center (DKFZ), Heidelberg, Germany, 3NYU Langone Medical Center, New York, NY, United States Motion-compensated images can be created from motion-binned undersampled radial stack-of-stars data through compressed sensing and image registration. However, for long repetition times or for many partitions, the acquisition time for one radial projection with all phase-encode steps becomes too long to sample the motion via self-gating, which leads to motion artifacts. Therefore, we estimate motion from FID-navigators and perform binning on a single-readout level to gain higher spatiotemporal resolutions. Our methods are tested on a motion phantom and volunteer with gridding and motion-compensated reconstructions. Our results show accurate detection of the motion signal and reduced motion blur in reconstructions. |
||
2348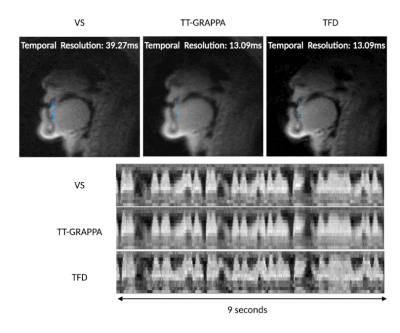 |
84 | Low Latency Real-Time MRI at 0.55T using Self-Calibrating Through-Time GRAPPA
Prakash Kumar1, Yongwan Lim1, and Krishna S. Nayak1
1Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States
Real-time MRI (RT-MRI) captures movements and dynamic processes in the human body as they occur, without reliance on any repetition or synchronization. Many applications of RT-MRI require low-latency reconstruction for feedback or interventions (typically <200ms). Here, we investigate self-calibrating spiral Through-Time GRAPPA at 0.55T and compare its performance against viewsharing and constrained reconstruction methods. We use longer readouts, which can be used at low-field due to reduced off-resonance effects. We demonstrate RT-MRI of speech production with 13.09ms temporal resolution and 25ms reconstruction latency (after a 30s pre-computed calibration step).
|
||
2349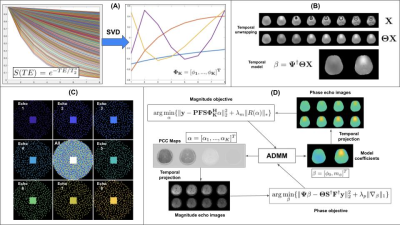 |
85 | Fast $$$T_{2}^{*}$$$ and QSM mapping using temporal CS with a Complementary Stochastic Sampling Scheme
Charles Iglehart1, Ali Bilgin1, and Manojkumar Saranathan2
1Electrical and Computer Engineering, University of Arizona, Tucson, AZ, United States, 2Department of Medical Imaging, University of Arizona, Tucson, AZ, United States
We develop the motivation for and demonstrate the functionality of a temporal model-based complex multi-echo reconstruction algorithm coupled with a Complementary Stochastic Sampling procedure allowing for temporal sparsity to be leveraged . We demonstrate results for magnitude and phase images as well as parameter maps.
|
||
2350 |
86 | Automatic reconstruction of arbitrary MRI sequences based on phase distribution graphs
Jonathan Endres1, Hoai Nam Dang2, Felix Glang3, Alexander Loktyushin3, Simon Weinmüller2, and Moritz Zaiss2,3
1Universitätsklinik Erlangen, Erlangen, Germany, 2Department of Neuroradiology, Universitätsklinik Erlangen, Erlangen, Germany, 3Magnetic Resonance Center, Max-Planck-Institute for Biological Cybernetics, Tübingen, Germany
We propose a method to automatically generate estimators for the exact encoding of a MRI signal, based on the Phase Distribution Graph of a sequence and its simulation. The estimator can then be used on a measurement to split the signal into its differently encoded parts, which enables reconstruction tailored to the sequence used. This approach can result in fewer imaging artifacts since it does not rely on any assumptions about the sequence made up front but on data obtained by a PDG simulation. This also makes it suitable for sequence optimization because it can adapt to changing sequence properties.
|
||
2351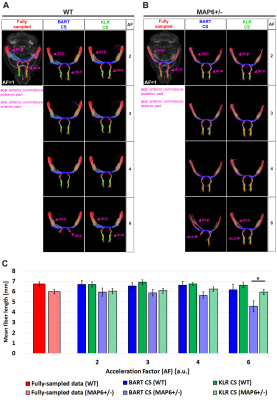 |
87 | Preclinical Evaluation of Two Compressed Sensing Methods for DTI
Diego Alves Rodrigues de Souza1, Hervé Mathieu1,2, Jean-Christophe Deloulme1, and Emmanuel L. Barbier1,2
1Univ. Grenoble Alpes - Inserm U1216 - Grenoble Institut des Neurosciences (GIN), Grenoble, France, 2Univ. Grenoble Alpes - Inserm US17 - CNRS UMS3552 - CHUGA - IRMaGe, Grenoble, France
Compressed Sensing (CS) is not routinely implemented at the preclinical level, especially in case of multiple receivers. In this study, we evaluate preclinical Kernel Low-Rank and Conventional CS reconstruction schemes at 9.4T using a 4-channel receive coil and spin-echo data. By analyzing diffusion parametric maps and white-matter tracts, we evaluate the median absolute error, the structural similarity index measure and the mean fiber length as a function of the acceleration factor and of the CS reconstruction pipeline.
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.