Digital Poster
White Matter & Gray Matter Microstructure in Health
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
1639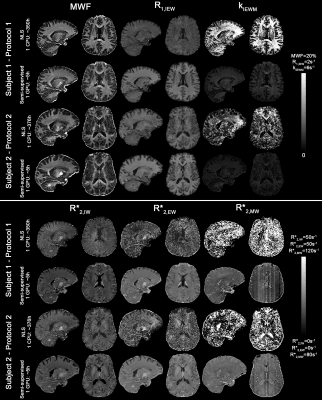 |
8 | Semi-supervised learning for fast multi-compartment relaxometry myelin water imaging (MCR-MWI)
Kwok-Shing Chan1, Tae Hyung Kim2,3, Berkin Bilgic2,3, and José P Marques1
1Donders Institute for Brain, Cognition and Behaviour, Nijmegen, Netherlands, 2Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Department of Radiology, Harvard Medical School, Boston, MA, United States Myelin water imaging using multi-compartment relaxometry (MCR-MWI) improves the GRE-MWI robustness and accuracy but suffered from slow processing speed. In this study, we incorporate both supervised and self-supervised machine learning for fast MCR-MWI that is generalisable to a wide range of acquisition parameters without the need to re-train the network. We demonstrate its application on single compartment fitting and MCR-MWI. Results show that the proposed method can produce comparable high SNR results with a 62-fold shorter processing time. |
||
1640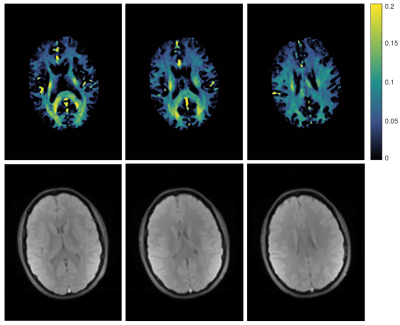 |
9 | In vivo myelin water imaging at 0.55T
Jessica Schäper1,2 and Oliver Bieri1,2
1Department of Biomedical Engineering, University of Basel, Basel, Switzerland, 2Division of Radioligical Physics, Department of Radiology, University of Basel Hospital, Basel, Switzerland
Myelin water fraction (MWF) imaging is an important tool to investigate demyelination in WM. When using GRE sequences, the method suffers from T2* effects, leading to a non-exponential behaviour. This can potentially influence the calculation of MWF. At low fields susceptibility effects are less and the assumption of biexponential T2-decay is more accurate. Here, the feasibility of MWF at 0.55 T, using biexponential fitting was demonstrated.
|
||
1641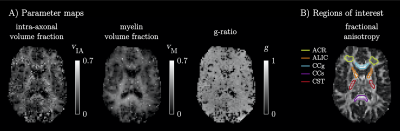 |
10 | Myelin-sensitive microstructure modeling of white matter using diffusion-T1-T2-relaxation MRI
Björn Lampinen1,2, Filip Szczepankiewicz3,4, and Markus Nilsson3
1Clinical Sciences Lund, Medical Radiation Physics, Lund University, Lund, Sweden, 2Skåne University Hospital, Lund, Sweden, 3Clincial Sciences Lund, Diagnostic Radiology, Lund University, Lund, Sweden, 4Brigham and Women's Hospital, Harvard Medical School, Boston, MA, United States
White matter pathology is characterized by demyelination and axonal loss. Diffusion MRI and relaxometry cannot separate these processes because they are primarily sensitive to axons and myelin, respectively. We used diffusion-T1-T2-relaxation MRI with tensor-valued encoding to support a twelve-parameter microstructure model including myelin. Results yielded brain parameter maps and values of the axonal and myelin volume fractions and the g-ratio that were in accordance with previous results from histology. The proposed approach can theoretically disambiguate between demyelination and axonal loss and could thus become a valuable tool for assessing disease severity and treatment response in pathologies that affect white matter.
|
||
1642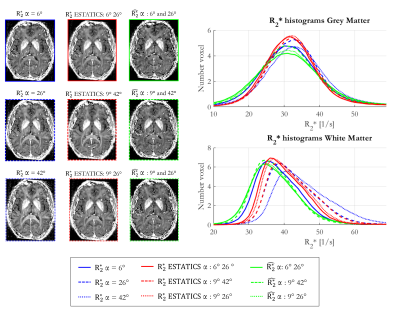 |
11 | How can we reliably mitigate flip angle and fibre orientation dependence in MPM-based R2* estimation at 7T?
Giorgia Milotta1, Nadège Corbin1,2, Christian Lambert1, Antoine Lutti3, Siawoosh Mohammadi4,5, and Martina Callaghan1
1Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 2Centre de Résonance Magnétique des Systèmes Biologiques, UMR5536, CNRS/University Bordeaux, Bordeaux, France, 3Laboratory for Research in Neuroimaging, Department for Clinical Neuroscience, , Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4Department of Systems Neurosciences, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 5Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
The apparent transverse relaxation rate ($$$R_{2}^{*}$$$) is influenced by biological features, e.g. iron and myelin content. However confounding factors, such as flip angle (α) and fibre orientation dependence, hinder interpretation. Multi-α data acquired as part of a comprehensive multi-parameter mapping approach can be used to mitigate these confounds. Here we explored how best to do so in vivo at 7T while additionally considering reproducibility. The ESTATICS approach, which assumes a common decay across flip angles, reduced these dependencies. $$$\hat{R_{2}^{*}}$$$, the α-independent component of a heuristic linear model, reduced both dependencies further but was less reproducible than ESTATICS.
|
||
1643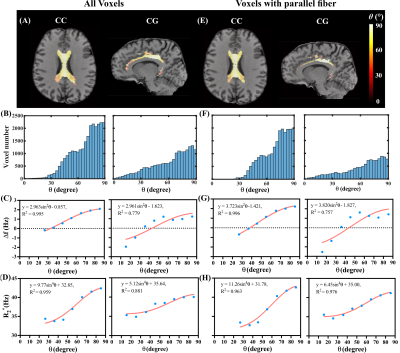 |
12 | Orientation dependence of local frequency difference and R2* in parallel versus crossing fibers at 3T and 7T
Lin Chen1,2, Deng Mao2, Guillaume Gilbert3, Maarten Versluis4, Peter van Zijl1,2, and Xu Li1,2
1Department of Radiology and Radiological Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3MR Clinical Science, Philips Canada, Mississauga, ON, Canada, 4MRI Clinical Scientist Neuroscience, Philips Healthcare, Best, Netherlands
We investigated the dependence of local frequency difference Δf and R2* on the fiber orientation with respect to the static field in selected parallel-fiber and crossing-fiber regions at 3T and 7T. Δf and R2* showed consistent relationship (sin2θ and sin4θ ) with average ROI-based fiber-to-field angle θ at 3T and 7T. Larger amplitude of variations in Δf and R2* with respect to θ were observed in parallel-fiber compared with crossing-fiber regions indicating non-negligible contributions from fiber orientation dispersion to the nonlinear gradient echo signal evolution and related MRI measures sensitive to white matter microstructure.
|
||
1644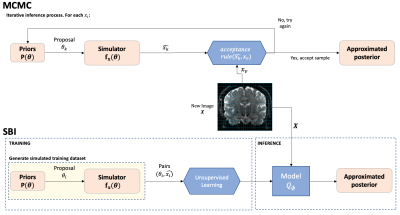 |
13 | Amortised inference in diffusion MRI biophysical models using artificial neural networks and simulation-based frameworks
Jose Pedro Manzano Patron1,2, Theodore Kypraios3, and Stamatios N Sotiropoulos4,5
1Sir Peter Mansfield Imaging Centre, Mental Health and Clinical Neurosciences, School of Medicine, University of Nottingham, Nottingham, United Kingdom, Nottingham, United Kingdom, 2Precision Imaging Beacon, University of Nottingham, Nottingham, United Kingdom, 3School of Mathematics, University of Nottingham, Nottingham, United Kingdom, 4Sir Peter Mansfield Imaging Centre, Mental Health and Clinical Neurosciences, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 5Wellcome Centre for Integrative Neuroimaging, University of Oxford, Oxford, United Kingdom Inference in imaging-based biophysical modelling provides a principled way of estimating model parameters, but also assessing confidence/uncertainty on results, quantifying noise effects and aiding experimental design. Traditional approaches in neuroimaging can either be very computationally expensive (e.g., Bayesian) or suitable to only certain assumptions (e.g., bootstrapping). We present a simulation-based inference approach to estimate diffusion MRI model parameters and their uncertainty. This novel framework trains a neural network to learn a Bayesian model inversion, allowing inference given unseen data. Results show a high level of agreement with conventional Markov-Chain-Monte-Carlo estimates, while offering 2-3 orders of magnitude speed-ups and inference amortisation. |
||
1645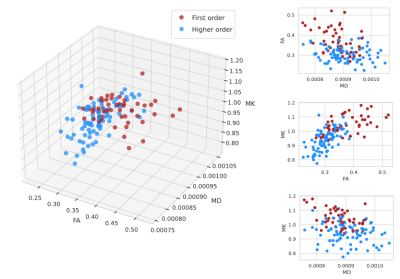 |
14 | In-vivo microstructural characterisation of first and higher order thalamic nuclei: a diffusion kurtosis imaging study Video Permission Withheld
Sebastian Hübner1, Lisa Novello1, Giacomo Tomezzoli1, and Jorge Jovicich1
1CIMeC, Center for Mind/Brain Sciences, University of Trento, Rovereto (Trento), Italy The thalamus is a primary station for information processing within the brain. It is composed of a collection of nuclei with different histological and functional properties, classified as First Order (FO, processing sensory input) or Higher Order (HO, playing associative roles). We used diffusion-weighted MRI (dMRI) to investigate microstructural properties of FO and HO thalamic nuclei, focusing on fractional anisotropy, mean diffusivity, and mean kurtosis derived from Diffusion Kurtosis Imaging (DKI). This initial evidence suggests that DKI might capture distinctive features of FO and HO thalamic nuclei, and paves the way to further studies investigating nucleus-specific microstructural effects with DKI. |
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.