Digital Poster
Susceptibility II
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
2456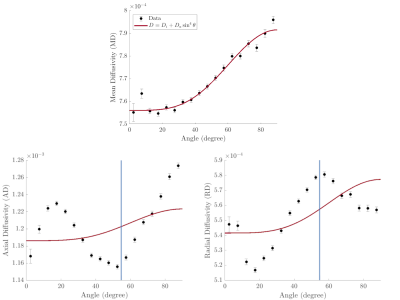 |
85 | Diffusion metrics in human white matter depend on fiber orientation
Lara Bartels1,2,3, Jonathan Doucette1,2,3, Christoph Birkl2,4, Alexander M. Weber2,3, and Alexander Rauscher1,2,3,5,6
1Physics & Astronomy, University of British Columbia, Vancouver, BC, Canada, 2MRI Research Centre, University of British Columbia, Vancouver, BC, Canada, 3Department of Pediatrics, University of British Columbia, Vancouver, BC, Canada, 4Department of Neuroradiology, Medical University of Innsbruck, Innsbruck, Austria, 5Department of Radiology, University of British Columbia, Vancouver, BC, Canada, 6Djavad Mowafaghian Centre for Brain Health, University of British Columbia, Vancouver, BC, Canada
In this work we studied the orientation dependence of diffusivities measured in DTI of healthy human white matter in vivo. The observed orientation dependence of the mean diffusivity can be explained by the effects of diffusion-mediated dephasing in the presence of diamagnetic myelin. Orientation dependence was markedly different for the radial and axial components of the diffusivity and suggests the presence of residual dipole-dipole interaction between water molecules.
|
||
2457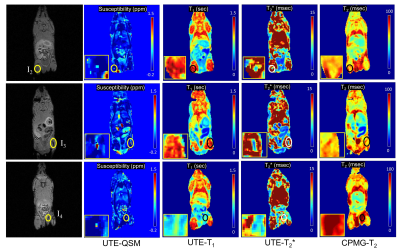 |
86 | Detection of Iron Oxide Nanoparticles (IONPs)-Labeled Stem Cells Using Quantitative UTE Imaging
Jiyo S Athertya1, Johnny Akers2, Sophia Dwek1, Zhao Wei1, Jiang Du1, Eric Y Chang1,3, Mya Thu2, and Hyungseok Jang1
1Radiology, University of California San Diego, San Diego, CA, United States, 2VisiCELL Medical Inc, San Diego, CA, United States, 3Radiology Service, VA San Diego Healthcare System, San Diego, CA, United States
Non-invasive, clinically applicable tracking of therapeutic cells by magnetic resonance imaging (MRI) offers unparalleled insight into the safety and efficacy of cell-based therapies in the body. Here we used a series of 3D quantitative UTE techniques including UTE-QSM, UTE-T1, and UTE-T2* mapping to evaluate the MR characteristics of stem cells labeled with a proprietary nanoparticle formulation that simultaneously labels cells for both optical and MR imaging. In a phantom experiment, all quantitative UTE parameters showed strong correlation with concentrations of labeled stem cells. Interestingly, in ex vivo mouse imaging, only UTE-QSM and UTE-T2* mapping detected the injected, labeled stem cells.
|
||
2458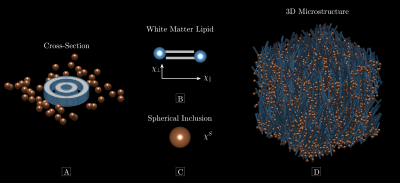 |
87 | The Larmor Frequency of a White Matter Magnetic Microstructure Model with Multiple Sources
Anders Dyhr Sandgaard1, Valerij G. Kiselev2, Noam Shemesh3, and Sune Nørhøj Jespersen1,4
1Department of Clinical Medicine, Center for Functionally Integrative Neuroscience, Aarhus University, Aarhus, Denmark, 2Medical Physics, Department of Radiology, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 3Champalimaud Research, Champalimaud Centre for the Unknown, Lisbon, Portugal, 4Department of Physics and Astronomy, Aarhus University, Aarhus, Denmark
Mapping tissue magnetic properties with MRI may improve the diagnosis of diseases and enhance our understanding of their basic mechanisms. However, MRI signals are sensitive to both structure and magnetic susceptibility, the so-called “magnetic microstructure”, rendering accurate susceptibility estimation a great challenge. Here, we present an analytical expression for the Larmor frequency of a white matter model of myelinated axons with axially symmetric microscopic susceptibility anisotropy and orientation dispersion. The modelled axons are surrounded by microscopic spherical inclusions with a scalar susceptibility. This goes beyond previous models of white matter magnetic microstructure.
|
||
2459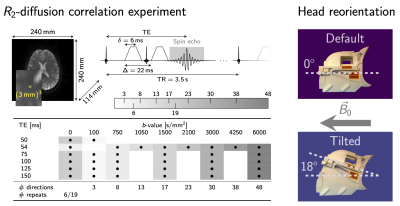 |
88 | The impact of head orientation with respect to B0: An unexplored source of variance in diffusion MRI
Elena Kleban1,2, Derek K Jones1,3, and Chantal MW Tax1,4
1CUBRIC, Cardiff University, Cardiff, United Kingdom, 2Inselspital, Bern University, Bern, Switzerland, 3Australian Catholic University, Melbourne, Australia, 4UMC Utrecht, Utrecht University, Utrecht, Netherlands
Recently observed anisotropy of compartmental white matter T2-values as a function of tissue orientation w.r.t. B0 in combination with echo-time-dependence of diffusion MRI signals suggest that similar tissue-orientational effects could be expected in standard diffusion tensor measures. In this work we show the change of up to 20-30% in diffusion tensor measures as a function of fibre orientation w.r.t. B0 from in vivo experiments and support these observations by simplified two-compartment white matter signal simulations.
|
||
2460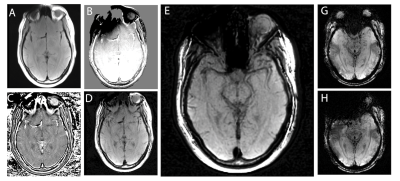 |
89 | Multi-Spectral Susceptibility-Weighted Imaging in the Presence of Metallic Hardware
Kevin Koch1, Brad Swearningen1, and Andrew S Nencka1
1Radiology, Medical College of Wisconsin, Milwaukee, WI, United States
To address the unmet need for metal artifact suppressed susceptibility-weighted imaging (SWI) in neuroradiology, here we introduce a novel approach to the generation of SWI contrasts using conventional (currently available) 3D-multi-spectral imaging (MSI) metal-artifact-suppression sequences. This approach leverages basic spin-echo based 3D-MSI sequences, and then utilizes the inherent spectral information within MSI to develop "phase-contrast" maps that can then used to generate SWI-like contrasts. This new metal-suppressed SWI approach has potential applications in patients with high susceptibility intra-cranial hardware, such as aneurysm clips, vascular shunts, cochlear implants, and fixed dental/orthodontic hardware.
|
||
2461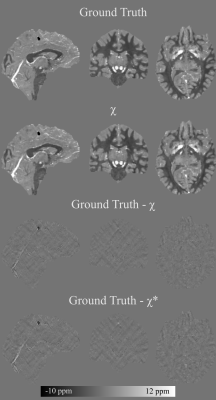 |
90 | Nonlinear multi-echo dipole inversion
Christian Kames1,2, Jonathan Doucette1,2, and Alexander Rauscher1,2,3
1UBC MRI Research Centre, University of British Columbia, Vancouver, BC, Canada, 2Department of Physics and Astronomy, University of British Columbia, Vancouver, BC, Canada, 3Department of Pediatrics, University of British Columbia, Vancouver, BC, Canada
We propose a two-pass multi-echo nonlinear dipole inversion method. In the first pass an initial susceptibility map is obtained. The susceptibility map is then further processed by deconvolving the residual of the forward computed phase and the input phase to recover remnant low frequency susceptibility sources. The proposed method attains a NRMSE of 18.8 on the QSM Reconstruction Challenge 2.0 calcification dataset (Sim2Snr1), decisively outperforming the best scoring submissions of the challenge (NRMSE 26.3, 28.3, 28.5).
|
||
2462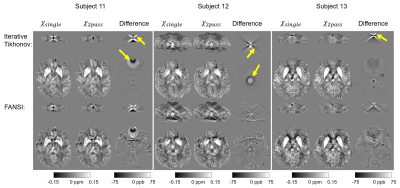 |
91 | A New, Simple Two-Pass Masking Approach for Streaking Artifact Removal in Any QSM Pipeline
Anita Karsa1 and Karin Shmueli1
1Medical Physics and Biomedical Engineering, University College London, London, United Kingdom
Tissue magnetic susceptibility maps calculated using any Quantitative Susceptibility Mapping (QSM) pipeline are often corrupted by streaking artifacts. Large streaking artifacts originating from extreme-susceptibility regions, such as interhemispheric calcifications or intracerebral bleeds, are common, not only in patients, but also in healthy, elderly subjects. Several variations on the two-pass masking approach have been proposed previously to suppress these artifacts. Here we propose a broadly-applicable two-pass masking method that is easy to implement and integrate into any QSM pipeline. We show that two-pass masking greatly reduces streaking from calcifications and cerebral bleeds without affecting susceptibility map anatomical features and values.
|
||
2463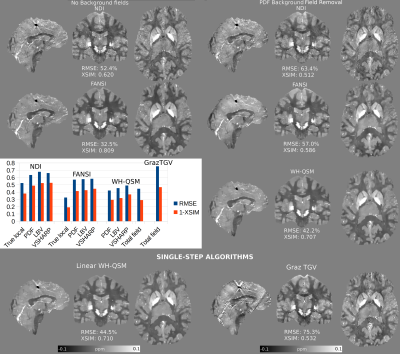 |
92 | A Comparison of Background Field Removal and “Single-Step” Algorithms in Realistic Phantoms: Towards QSM Challenge 3.0?
Carlos Milovic1, Christian Langkammer2, José Pedro Marques3, and Karin Shmueli1
1Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 2Department of Neurology, Medical University of Graz, Graz, Austria, 3Donders Centre for Cognitive Neuroimaging, Radboud University, Nijmegen, Netherlands
The 2019 QSM Reconstruction Challenge compared how different algorithms reconstructed QSM from true local field maps. However, this neglects the importance of residual background fields, and how they degrade the solutions. Here, we used a realistic “total field” simulation to compare three different background field removal methods, and to estimate the susceptibility maps resulting from three state-of-the-art QSM algorithms. We also compared the performance of two single-step algorithms, that use the total field map as an input. Our results showed that the two-step Weak-Harmonic QSM method is robust against residual fields and achieved the lowest error scores, outperforming single-step methods.
|
||
2464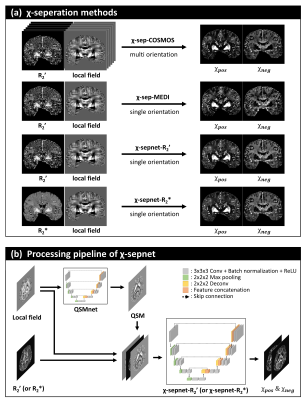 |
93 | Chi-sepnet: Susceptibility source separation using deep neural network
Minjun Kim1, Hyeong-Geol Shin1, Chungseok Oh1, Hwihun Jeong1, Sooyeon Ji1, Hongjun An1, Jiye Kim1, Jinhee Jang2, Berkin Bilgic3, and Jongho Lee1
1Seoul National University, Seoul, Korea, Republic of, 2Seoul St Mary’s Hospital, Seoul, Korea, Republic of, 3Harvard Medical School, Boston, MA, United States The separation of positive and negative susceptibility source distributions (e.g., iron and myelin distributions) has important meanings in neuroscience and clinic. In this study, a deep learning-based χ-separation method is proposed to generate high-quality susceptibility source maps. For network training, multi-orientation head data are utilized, providing artifact-free label data. For the input data, either R2’ or R2* maps are utilized in addition to local field and QSM maps, producing two neural networks, χ-sepnet-R2’ and χ-sepnet-R2* (the latter requires no T2). The results of χ-sepnets outperformed the conventional method, revealing details of brain structures both in healthy volunteers and patients. |
||
2465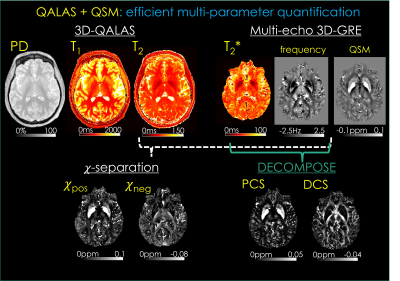 |
94 | QALAS + QSM: Efficient Multi-parameter Mapping Allows Disentangling Para- and Dia-magnetic Contributions in Brain Tissue
Hyeong-Geol Shin1, Jingjia Chen2, Jongho Lee1, Tae Hyung Kim3, Jaejin Cho3, Gabriel Varela-Mattatall3, Borjan Gagoski4, Chunlei Liu2, and Berkin Bilgic3
1Department of Electrical and Computer Engineering, Seoul National University, Seoul, Korea, Republic of, 2Department of Electrical Engineering and Computer Sciences, University of California, Berkeley, Berkeley, CA, United States, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 4Fetal-Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, Boston, MA, United States Quantitative MRI has demonstrated potential in clinical and neuroscience applications, but its adoption has been hampered by excessively long scan times. Recent biophysical models have enabled differentiation of para- and dia-magnetic contributions to the tissue, but the acquisition of multi-parametric maps required by such models have further increased the encoding burden. We propose a comprehensive 9.5 min exam at high isotropic resolution to address this, from which T1, T2, proton density, T2*, para- and dia-magnetic susceptibility maps are estimated. This exam flexibly lends itself to generation of contrast-weighted images, allowing it to be used for clinical reads as well. |
||
2466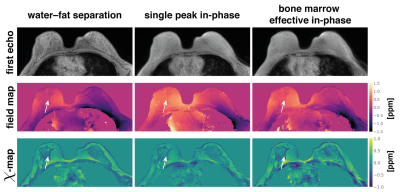 |
95 | On the water–fat in-phase assumption for quantitative susceptibility mapping (QSM)
Christof Boehm1, Jakob Meineke2, Kilian Weiss3, Marcus R Makowski1, and Dimitrios C Karampinos1
1Department of Diagnostic and Interventional Radiology, School of Medicine, Klinikum Rechts der Isar, Technical University of Munich, Munich, Germany, 2Philips Research, Hamburg, Germany, 3Philips GmbH Market DACH, Hamburg, Germany
Gradient echo imaging using in-phase echoes has been proposed to reduce the field-map estimation in water–fat regions to a convex nonlinear least squares problem. Conventionally, the in-phase assumption is based on a single-peak fat-model. However, fat is known to have a complex spectrum rendering the definition of in-phase echo times problematic. In this work, the single-fat-peak in-phase assumption is replaced by a multi-peak effective in-phase assumption. QSM based on multi-peak in-phase echo times is shown to yield similar results to water–fat imaging based field- and susceptibility-mapping in a simulation and in vivo in the spine and the breast.
|
||
2467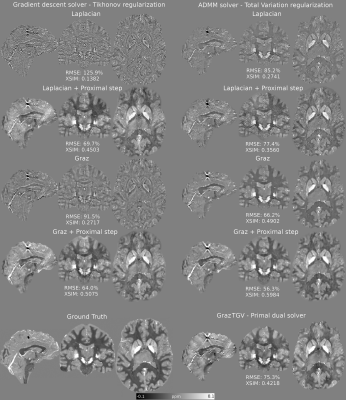 |
96 | A Proximal Step Enables Fast and Accurate Single-Step QSM Reconstructions, Preventing Susceptibility Underestimation
Carlos Milovic1, Kristian Bredies2, Christian Langkammer3, and Karin Shmueli4
1University College London, London, United Kingdom, 2Institute of Mathematics and Scientific Computing, University of Graz, Graz, Austria, 3Department of Neurology, Medical University of Graz, Graz, Austria, 4Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom
Single-step quantitative susceptibility mapping (QSM) algorithms simplify the processing pipeline and promise to be more robust against background fields than traditional two-step methods but they often underestimate tissue susceptibilities. Here, we propose a highly efficient gradient descent Tikhonov-regularized proximal solver and a highly accurate ADMM TV-regularized proximal solver to improve the accuracy of two Laplacian-based single-step methods. Our solvers outperformed current single-step methods and showed in-vivo performance very similar to traditional two-step methods. This will simplify QSM processing pipelines, allowing further automation in future, although more research is needed to improve robustness against noise and boundary-conditions-related artifacts.
|
||
2468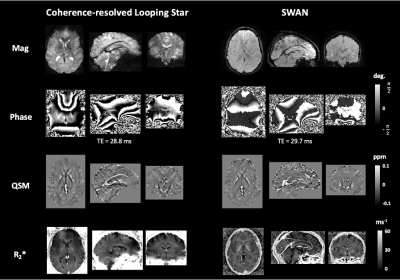 |
97 | Quiet quantitative susceptibility mapping with Looping Star
Nikou Louise Damestani1, Ana Beatriz Solana2, Florian Wiesinger2, Brice Fernandez3, Steven Charles Rees Williams1, and David John Lythgoe1
1Department of Neuroimaging, King's College London, London, United Kingdom, 2GE Healthcare, Munich, Germany, 3GE Healthcare, Paris, France
Looping Star is an acoustically quiet T2*-weighted acquisition technique, which has primarily been used to demonstrate functional sensitivity. We present the application of Looping Star to quantitative susceptibility mapping in a small cohort, directly comparing its outcomes with a conventional susceptibility-weighted imaging technique. We found that Looping Star produced comparable quantitative values in subcortical regions in comparison with conventional susceptibility-weighted imaging.
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.