Digital Poster
CEST: Cancer
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
2799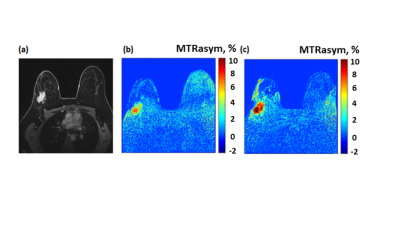 |
67 | Molecular imaging of breast cancer by chemical exchange saturation transfer (CEST) MRI of glucosamine: first human experience
Michal Rivlin1, Debbie Anaby2,3, Noam Nissan2,3, Moritz Zaiss4, Anagha Deshmane5, Miri Sklair-Levy3,6, and Gil Navon1
1School of Chemistry, Tel-Aviv University, Tel-Aviv, Israel, 2Department of Diagnostic Imaging, Sheba Medical Center, Ramat-Gan, Israel, 3The Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel, 4Department of Neuroradiology, University Clinic Erlangen, Friedrich-Alexander Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 5Department of Biomedical Magnetic Resonance, University of Tübingen, Tübingen, Germany, 6Meirav High Risk Clinic, Department of Diagnostic Imaging, Sheba Medical Center, Ramat-Gan, Israel
In preclinical experiments in implanted breast cancer tumors in mice, glucosamine (GlcN) exhibited enhanced chemical exchange saturation transfer (CEST) MRI signals. Moving toward clinical application, considering the excellent safety profile of GlcN, we examined the feasibility of using the GlcN CEST method to detect human breast cancer on a 3T clinical scanner. Here we report significant CEST MRI signals resulting from the exchangeable protons of GlcN hydroxyls, amine/amide residues as well as nuclear Overhauser enhancement (NOE). Thus, CEST MRI using GlcN has the potential to detect tumors and report their activity, without the use of a gadolinium contrast agent.
|
||
2800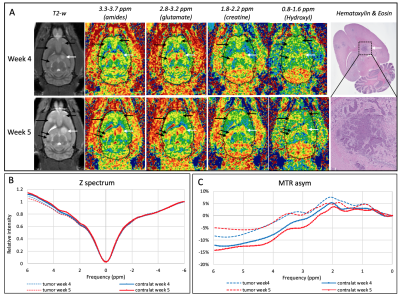 |
68 | In vivo metabolic CEST changes in brain tumors in a model of metastatic breast cancer
Jennifer Lefeuvre1, Katie MacKey1, Tsang-Wei Tu2, and Joseph Frank1
1Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD, United States, 2Molecular imaging laboratory, Department of Radiology, Howard University College of Medicine, Washington DC, DC, United States
Metabolic changes may occur earlier and/or may help distinguish different type of tumors. We investigated the metabolic changes using CEST MRI and characterized induced metastatic breast cancer in the brain of 10 nude rats. In vivo CEST MRI were performed at 9.4T on 3 slices with 31 frequency offsets to cover the full Z-spectra. A total of 12 tumors were analyzed for CEST signal changes. Different tumor types (solid tumor vs necrosis) showed different metabolites uptakes. Tumors located within the dentate gyrus showed significant increased values compared to the tumors in brainstem, cortex and hippocampus.
|
||
2801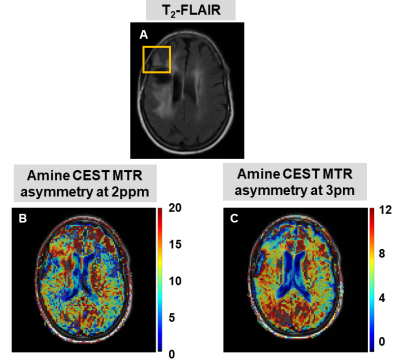 |
69 | A preliminary investigation into the contribution of Amines to the CEST contrast at 2 ppm and 3 ppm in high-grade gliomas at 7T
Bárbara Schmitz Abecassis1, Chloé Najac1, Jeroen de Bresser 1, Linda Dirven2,3, Martin J.B. Taphoorn2,3, Matthias J.P. van Osch1, Johan A.F. Koekkoek2,3, and Ece Ercan1
1Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 2Department of Neurology, Leiden University Medical Center, Leiden, Netherlands, 3Department of Neurology, Haaglanden Medical Center, The Hague, Netherlands
CEST allows to non-invasively image metabolites and proteins, and the role of APT and NOE CEST in gliomas has been widely investigated. On the other hand, the contribution of amines to the CEST signal at 2 and 3 ppm, and its relationship to creatine and glutamate concentrations, remain unclear. We evaluated the amine CEST contrast in tumor and contralateral white matter in three glioma patients, and compared it to the MRS data. Overall we found a decrease in amine CEST alongside a decrease in total creatine and glutamate in the tumor compared to the contralateral white matter.
|
||
2802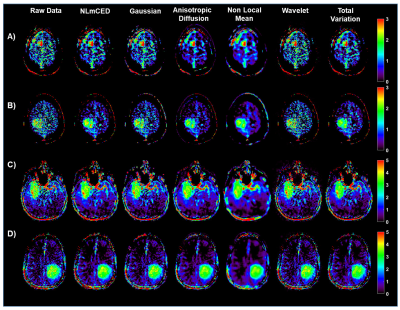 |
70 | Improving clinical 3T Amide Proton Transfer weighted contrast in Brain Tumors by using a novel spatial denoising method
Feriel Romdhane1, Dario Livio Longo1, Christos Papageorgakis2, Eleni Firippi2, Laura Mancini3,4, Sotirios Bisdas3,4, Moritz Zaiss5, and Stefano Casagranda2
1Institute of Biostructures and Bioimaging (IBB), National Research Council of Italy (CNR), Torino, Italy, 2Department of Research & Innovation, Olea Medical, La Ciotat, France, 3Lysholm Department of Neuroradiology, University College of London Hospitals NHS Foundation Trust, London, United Kingdom, 4Institute of Neurology UCL, London, United Kingdom, 5Department of Neuroradiology, University Clinic Erlangen, Friedrich-Alexander Universität Erlangen-Nürnberg (FAU), Erlangen, Germany Amide Proton Transfer weighted (APTw) imaging is a promising MR molecular technique for characterizing brain tumors on clinical 3T scanners. However, improving the APT weighted contrast is crucial to translate this approach to clinical practice. In this work we investigated a novel spatial denoising filter in synthetic and clinical APTw data of brain tumor patients. |
||
2803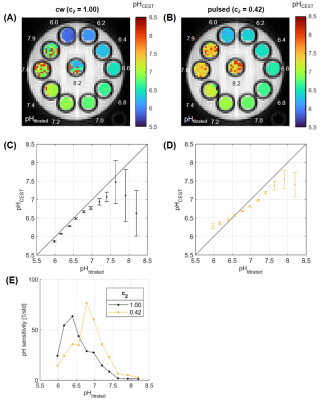 |
71 | Presaturation pulse shape enables shifting the pH sensitivity of guanidyl CEST-MRI for absolute pH mapping at 9.4 T
Philip S Boyd1,2, Lilli Diederichs1,2, Johannes Breitling1, Mark E Ladd1,2,3, Peter Bachert1,2, and Steffen Goerke1
1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, University of Heidelberg, Heidelberg, Germany, 3Faculty of Medicine, University of Heidelberg, Heidelberg, Germany In this study, we present a method for absolute pH mapping using the guanidyl CEST signal. The method is an extension of a previous approach that allowed compensating for various concomitant effects other than pH, but additionally required measuring the amide signal. By optimizing the pulse shape of the pulsed presaturation the pH sensitivity of the guanidyl signal could now be shifted to the physiologically relevant range around pH 7.1. The shift of sensitivity was verified experimentally in a multi-pH creatine phantom at 9.4T. Thus, CEST-based pH mapping with exceptional specificity is now also possible using only the guanidyl signal. |
||
2804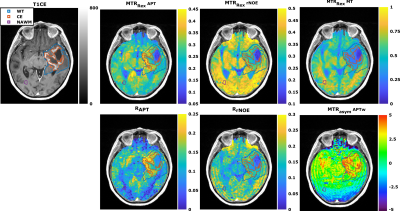 |
72 | Comparison study between APT-weighted and relaxation-compensated CEST-MRI in human glioma at 3T
Florian Kroh1,2, Johannes Breitling1, Nikolaus von Knebel Doeberitz3, Heinz-Peter Schlemmer3,4, Mark E. Ladd1,2,4, Peter Bachert1,2, Daniel Paech3,5, and Steffen Goerke1
1Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, University of Heidelberg, Heidelberg, Germany, 3Division of Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Faculty of Medicine, University of Heidelberg, Heidelberg, Germany, 5Division of Neuroradiology, University Hospital Bonn, Bonn, Germany Although the underlying mechanisms of APT-weighted (APTw) CEST-MRI are not completely understood, it has been shown to provide valuable information for brain tumor imaging at 3T. To gather more information about the mechanisms, the APTw signal was correlated to relaxation-compensated CEST contrasts in 21 postoperative human glioma patients. A correlation, although being only moderate, was found for the relaxation-compensated APT contrast in the contrast enhancing (CE) tumor region, but not for other tissues, nor rNOE or MT contrast. Differentiation of the CE tumor and normal appearing white matter was strongly dependent on the selective relaxation-compensated CEST evaluation method. |
||
2805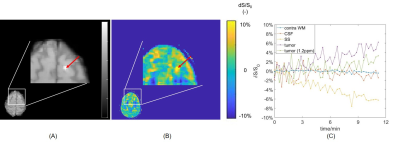 |
73 | Dynamic Glucose Enhanced (DGE) CEST imaging at 3T: from phantom to patient with brain metastases Video Permission Withheld
Yulun Wu1, Sophie H.A.E. Derks1,2,3, Tobias Wood4, Astrid A.M. van der Veldt1,2, Marion Smits1, and Esther A.H. Warnert1
1Department of Radiology & Nuclear Medicine, Erasmus MC, Rotterdam, Netherlands, 2Department of Medical Oncology, Erasmus MC, Rotterdam, Netherlands, 3Department of Neurology, Erasmus MC, Rotterdam, Netherlands, 4Centre for Neuroimaging Science, King's College London, London, United Kingdom To develop detection of glucose contrast enhancement (GCE) with CEST for assessing brain metastases in clinical setting, here we performed a phantom study, scanned 10 healthy volunteers and one patient with brain metastases at 3T. We acquired dynamic GCE at 1.2 and 2 ppm for dynamic B0 correction and applied principle component analysis (PCA) to perform noise reduction, after which we compared the resulting GCE at both offsets. The developed pipeline resulted in GCE of ~5% in tumor and -5 to 0.05% in healthy tissues after glucose infusion. |
||
2806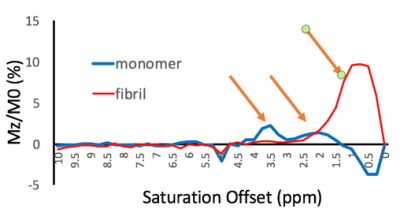 |
74 | Healthy vs pathological tau can be differentiated by CEST NMR
Fang Frank Yu1, James Ratnakar2, Jonathan A Brewer1, Brian Hitt3, A Dean Sherry1,2, and Elena Vinogradov1,2
1Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Advanced Imaging Reseach Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Neurology, University of California, Irvine Medical Center, Irvine, CA, United States
Alzheimer’s Disease (AD) is defined biologically by the presence of neuritic plaques composed of amyloid-in β (Aβ)peptides and dystrophic neurites, neurofibrillary tangles (NFTs) of hyperphosphorylated tau protein, and neuronal loss. In this study, CEST experiments were performed on purified full-length tau monomer and aggregated tau fibrils. We found differences in CEST z-spectra between purified full-length tau monomers and aggregated tau fibrils. This finding substantiates the potential use of CEST for ultimately monitoring disease progression in AD patients
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.