Digital Poster
Hybrid Systems, Magnets, Gradient Systems & Shims/Shimming I
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Computer # | ||||
|---|---|---|---|---|
1287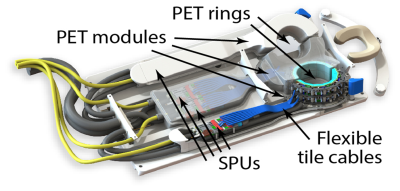 |
83 | The HYPMED PET/MRI Insert for Breast Cancer
Yannick Kuhl1, Stephan Naunheim1, Bjoern Weissler1,2, Vanessa Nadig1, David Schug1,2, Florian Mueller1, Harald Radermacher1, Pierre Gebhardt1, Nicolas Gross-Weege1, Teresa Nolte1, Martino Borgo3, Marcel de Koning3, Menno Mathlener3, Jeroen Koeleman3, Frank van Duin3, Arold Dekker3, Wout Schut3, Jos van den Berghe3, Daniel Gareis4, Turgay Celik4,
André Salomon5, Dennis Schaart6, Dimitri Kuznetsov6, René Bakker6, Karl-Josef Langen7, Christiane Kuhl8, and Volkmar Schulz1,2,9,10
1Physics of Molecular Imaging Systems (PMI), Experimental Molecular Imaging (ExMI), Aachen, Germany, 2Hyperion Hybrid Imaging Systems GmbH, Aachen, Germany, 3Futura Composites B.V., Heerhugowaard, Netherlands, 4NORAS MRI Products GmbH, Hoechberg, Germany, 5Philips Research, Eindhoven, Netherlands, 6Delft University of Technology, Delft, Netherlands, 7Forschungszentrum Juelich, Juelich, Germany, 8Department of Diagnostic and Interventional Radiology, University Hospital Aachen, Aachen, Germany, 9Physics Institute III B, RWTH Aachen University, Aachen, Germany, 10Fraunhofer Institute for Digital Medicine MEVIS, Aachen, Germany
Within the H2020 EU project HYPMED, we have developed a PET insert that can be moved into a 1.5 T MRI Philips Ingenia turning this into a simultaneous MRI-PET device for breast cancer applications. The PET insert consists of two independent PET rings with a field of view of 28$$$\times$$$10 cm² and two dual-channel local receive coils. Each ring consists of 14$$$\times$$$2 detector blocks of which each features a three-layer LYSO crystal array with 1.3 mm pitch. We will present the first PET and MRI results of our system.
|
||
1288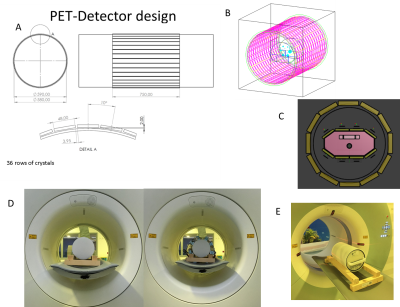 |
84 | Feasibility study of novel 8-channel stacked 1H/31P transceiver coil as PET insert for 7T MRI Video Permission Withheld
Ria Forner1, Woutjan Branderhorst1, Debra Rivera2, Alexander Raaijmakers1,2, Dimitri Welting1, and Dennis Klomp1
1UMC Utrecht, Utrecht, Netherlands, 2Eindhoven University of Technology, Eindhoven, Netherlands
The feasibility of simultaneous 1H/31P PET/MR imaging added-on to an existing 7T MRI system was assessed. Space constraints preclude using whole-body birdcages. Instead, an 8-channel 1H dipole array stacked with additional 8-31P channels is set up. By positioning PET detectors between the RF-shield and gradient coil, further shielding of the PET-crystals and electronics can be omitted. 8-channel 31P scans had a higher SNR compared to a birdcage setup. The 1H/31P setup gives ~5% degradation of PET sensitivity. Similarly, the impact of the new setup (proximity of the RF shield) was insignificant for MRI performance at both frequencies.
|
||
1289 |
85 | A Novel PET Optimized Head Neck Coil for 3T Simultaneous MRPET System
Yun-Jeong Stickle1, Mark Giancola1, Clyve Konrad Follante1, Thomas Stickle1, Fraser Robb1, Holly Blahnik2, and Tae-Young Yang1
1GE Healthcare, Aurora, OH, United States, 2GE Healthcare, Waukesha, WI, United States
A PET optimized 21- Element Head Neck coil is described for acquiring high-sensitivity MR (Magnetic Resonance) images and good quality PET images of head/neck, carotids, and cervical spine at 3 Tesla simultaneous MR/PET system. This Head Neck prototype2 coil has been optimized to minimize the interference with -ray detection of the PET without sacrificing MR image quality. In this study, we developed a foam technology to replace the rigid plastic coil formers, optimized arrangement of components (internal cables, internal cable baluns, feed boards and decoupling boards) and introduced new mechanical fastener and snap features.
|
||
1290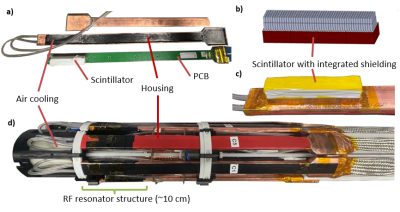 |
86 | MRI compatibility for a novel simultaneous PET/MRI system with scintillator-based RF shielding for preclinical 7 T MRI scanner Video Permission Withheld
Laiyin Yin1, Nicolas Gross-Weege1, Teresa Nolte1, Bjoern Weissler1,2, David Schug1,2, and Volkmar Schulz1,2,3,4
1Department of Physics of Molecular Imaging Systems, Institute of Experimental Molecular Imaging, RWTH Aachen University, Aachen, Germany, 2Hyperion Hybrid Imaging Systems GmbH, Aachen, Germany, 3Physics Institute III B, RWTH Aachen University, Aachen, Germany, 4Fraunhofer Institute for Digital Medicine MEVIS, Aachen, Germany
We present a preclinical PET/MRI system with highly-integrated PET modules in which the RF shielding is implemented on scintillator level for a 7 T Bruker BioSpec 70/20. The PET system's RF housing size is minimized by excluding parts of the scintillator while maintaining the RF shielding properties through the scintillator-integrated shielding. The modules are mounted inside the MRI coil, between resonator and RF screen. MRI compatibility is evaluated in a mouse phantom by means of spurious noise scans and SNR evaluations of standard morphologic sequences, while the PET system is off, idle and measuring. Additionally, postmortem mouse images are presented.
|
||
1291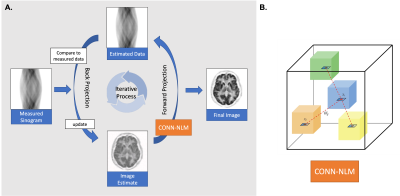 |
87 | MRI tractography-guided PET image reconstruction regularisation using connectome-based nonlocal means filtering
Zhuopin Sun1, Georgios Angelis1,2, Steve Meikle3,4, and Fernando Calamante1,4,5
1School of Biomedical Engineering, The University of Sydney, Sydney, Australia, 2Australian National Imaging Facility, The University of Sydney, Sydney, Australia, 3Faculty of Medicine and Health, The University of Sydney, Sydney, Australia, 4Brain and Mind Centre, The University of Sydney, Sydney, Australia, 5Sydney Imaging, The University of Sydney, Sydney, Australia
Diffusion MRI (dMRI) can provide a wealth of information about brain microstructure and connectivity, but its use in PET-MRI image reconstruction has not been exploited. We incorporated dMRI-derived prior information as a regulariser into the iterative PET One-Step Late Maximum A Posteriori (OSLMAP) reconstruction algorithm. The proposed regularisation method has unique advantages of providing more informative and targeted denoising and regularisation based on the complementary structural connectivity information from dMRI.
|
||
1292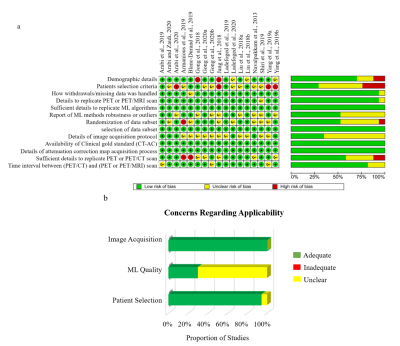 |
88 | An Evaluation of the Diagnostic Quality of Machine Learning Approaches for PET Attenuation Correction in Neuroimaging: A Meta-Analysis
Confidence Raymond1,2, Jurkiewicz Michael 1,2, Akin Orunmuyi3, Dada Oluwaseun Michael 4, Claes Nøhr Ladefoged5, Jarmo Teuho6, and Udunna Anazodo1,2
1Medical Biophysics, Western University Ontario, London, ON, Canada, 2Lawson Health Research Institute, LONDON, ON, Canada, 3Anaesthesia, College of Medicine, Ibadan, Nigeria, 4Physics, Federal University of Technology, Minna, Nigeria, 5Clinical Physiology, Nuclear Medicine and PET, Rigshospitalet, Denmark, 6Turku PET Centre, Turku University, Turku, Finland
The last decade has seen an increase in the application of machine learning (ML) methods to PET/MRI attenuation correction (AC). This systematic review provides a head-to-head comparison between state-of-the-art ML methods and clinical standards for AC to determine the clinical feasibility of ML approaches PET AC. We extracted numerical values for image quality, tissue classification, regional and global diagnostic performance. The pooled mean relative error for global performance was 0.87 ± 1.3%, the quality of evidence for all outcomes ranged from moderate to very low. Our findings suggest that ML-AC performance is within acceptable limits for clinical PET/MR neuroimaging.
|
||
1293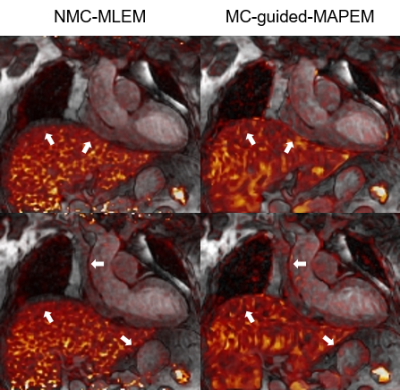 |
89 | Non-rigid Motion-compensated Whole-heart 18F-FDG PET and 3D T2 mapping in a hybrid PET-MR system
Alina Schneider1, Camila Munoz1, Alina Hua1, Sam Ellis1, Sami Jeljeli2, Karl P Kunze1,3, Radhouene Neji1,3, Eliana Reyes1, Tevfik F Ismail1, René M Botnar1, and Claudia Prieto1
1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2PET Centre, St Thomas’ Hospital, King’s College London & Guys and St Thomas’ NHS Foundation Trust, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom Simultaneously acquired 18F-FDG PET-MR imaging and quantitative 2D T2-mapping have been suggested for improved diagnostic accuracy of cardiac sarcoidosis, however misregistration between imaging modalities makes clinical interpretation challenging. Here we used a recently proposed free-breathing motion-corrected 3D whole-heart T2-mapping sequence acquired simultaneously with 18F-FDG PET at a 3T PET-MR system. This approach enables the non-rigid motion-correction for both the 3D T2-mapping and the PET data to the same respiratory position, resulting in aligned volumes for improved clinical interpretation. In this study, we tested this approach in a patient with suspected cardiac sarcoidosis. |
||
1294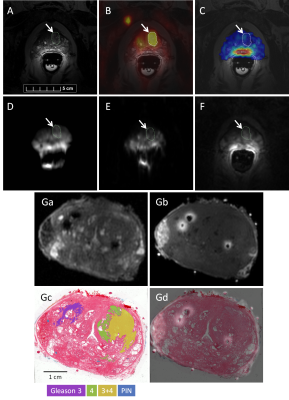 |
90 | Multiparametric MRI, sodium MRI, and PSMA PET of prostate cancer with histological validation of Gleason grade
Josephine Tan1, Alireza Akbari1, Matthew Fox2, Mena Gaed3, Madeleine Moussa4, Jose A Gomez4, Zahra Kassam5, William Pavlosky5, Joseph L Chin6, Stephen Pautler6, Nicholas Power6, Aaron D Ward1,2, Glenn S Bauman1,2, Jonathan D Thiessen1,2,5, and Timothy J Scholl1,3,7
1Medical Biophysics, University of Western Ontario, London, ON, Canada, 2Lawson Health Research Institute, London, ON, Canada, 3Robarts Research Institute, University of Western Ontario, London, ON, Canada, 4Pathology and Laboratory Medicine, University of Western Ontario, London, ON, Canada, 5Medical Imaging, University of Western Ontario, London, ON, Canada, 6Surgery, Division of Urology, University of Western Ontario, London, ON, Canada, 7Ontario Institute for Cancer Research, Toronto, ON, Canada
This abstract focuses on the development of an imaging assay based on multiparametric MRI and sodium (23Na) MRI to discriminate between low- and high-grade prostate cancer (PCa) lesions, using Gleason grade defined by whole-mount histopathology as the gold standard and PET targeting the prostate-specific membrane antigen (PSMA) as a validation. Data from the first patient (Gleason score 7) demonstrates that PSMA-PET may be superior in detecting PCa lesions in the transition zone and distant metastases while the sensitivity of the current 23Na radiofrequency system in this study is limited to lesions in the peripheral zone of the prostate.
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.