Online Gather.town Pitches
Data Acquisition & Artefacts II
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| Booth # | ||||
|---|---|---|---|---|
5008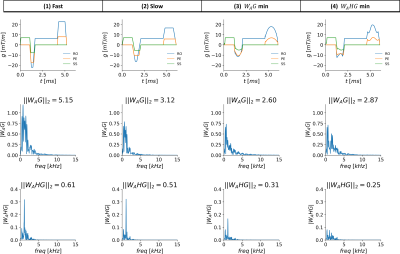 |
1 | Acoustic Frequency Minimization of Gradient Waveforms with GrOpt
Michael Loecher1,2 and Daniel B Ennis1,2
1Radiology, Stanford University, Stanford, CA, United States, 2Radiology, Veterans Administration Health Care System, Palo Alto, CA, United States
In this work, we measured the acoustic frequency response function of an MRI scanner. The response was then used to design arbitrarily shaped gradient waveforms that minimize the predicted acoustic noise output of the sequence. Two minimization functions were tested and compared to the conventional sequence with two different slew rates. The method was used to generate a GRE sequence with 31% reduced acoustic output for a 17% increase in scan time or 16% reduced acoustic output compared to the slew rate derated sequence, both with no difference in image quality.
|
||
5009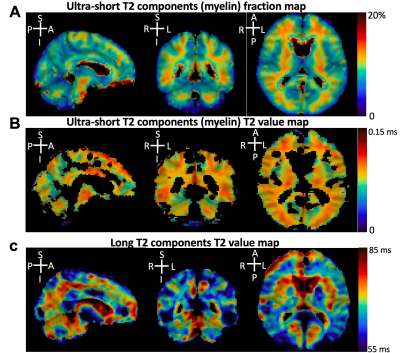 |
2 | Myelin Imaging Using 3D Dual-echo Ultra-short Echo Time MRI with Rosette k-Space Pattern
Xin Shen1, Ali Caglar Özen2, Antonia Sunjar1, Serhat Ilbey2, Riyi Shi1,3, Mark Chiew4, and Uzay Emir1,5
1Weldon School of Biomedical Engineering, Purdue University, West Lafayette, IN, United States, 2Department of Radiology, Medical Physics, University of Freiburg, Freiburg, Germany, 3College of Veterinary Medicine, Purdue University, West Lafayette, IN, United States, 4Wellcome Centre for Integrative Neuroimaging, University of Oxford, Oxford, United Kingdom, 5Health Science Department, Purdue University, West Lafayette, IN, United States
This study aimed to develop a new 3D dual-echo rosette k-space trajectory, specifically for applications of ultra-short echo time (UTE) magnetic resonance imaging (MRI). The direct imaging of the myelin bilayer, which has ultra-short transverse relaxation time (uT2), was acquired to test the performance of the proposed UTE sequence. The rosette trajectory was developed based on rotations of a ‘petal-like’ pattern in the kx-ky plane, with oscillated extensions in kz-direction for 3D coverage. The higher uT2 fraction value in white matter (WM) compared to grey matter (GM) demonstrated the ability of the proposed sequence to capture rapidly decaying signals.
|
||
5010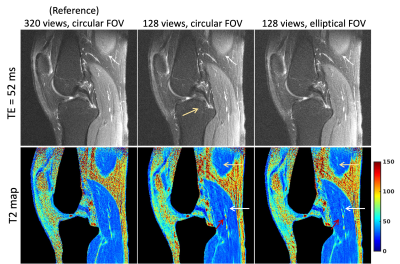 |
3 | Elliptical Field-of-Views in Radial T2 mapping
Zhiyang Fu1,2, Ute Goerke1,3, Kevin Johnson1, Ali Bilgin1,2,4, and Maria Altbach1,4
1Medical Imaging, The University of Arizona, Tucson, AZ, United States, 2Electrical and Computer Engineering, The University of Arizona, Tucson, AZ, United States, 3Siemens Medical Solutions USA Inc, The University of Arizona, Tucson, AZ, United States, 4Biomedical Engineering, The University of Arizona, Tucson, AZ, United States
Radial imaging is appealing for quantitative parameter mapping, due to their inherently robustness to undersampling compared to Cartesian trajectories and thus, its ability to yield parameter maps with high spatial resolution from highly undersampled data. Two-dimensional radial imaging typically acquires equiangular spaced lines, resulting in a circular field-of-view (FOV). Circular FOVs are not ideal for imaging applications with an anisotropic region-of-support (e.g., spine, leg). Larson et al. proposed an algorithm for designing fully sampled radial trajectories matching the prescribed anisotropic FOV. In this work we investigate the benefits of anisotropic FOV in radial MRI in the context of parameter mapping.
|
||
5011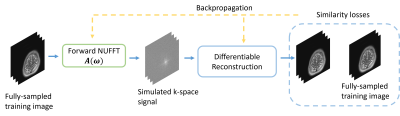 |
4 | Reconstruction May Benefit from Tailored Sampling Trajectories: Optimizing Non-Cartesian Trajectories for Model-based Reconstruction
Guanhua Wang1, Douglas C. Noll1, and Jeffrey A. Fessler2
1Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States, 2EECS, University of Michigan, Ann Arbor, MI, United States
This abstract explores non-Cartesian sampling trajectories that are optimized specifically for various reconstruction methods (CG-SENSE, penalized least-squares, compressed sensing, and unrolled neural networks). The learned sampling trajectories vary in the k-space coverage strategy and may reflect underlying characteristics of the corresponding reconstruction method. The reconstruction-specific sampling trajectory optimization leads to the most reconstruction quality improvement. This work demonstrates the potential benefit of jointly optimizing imaging protocols and downstream tasks (i.e., image reconstruction).
|
||
5012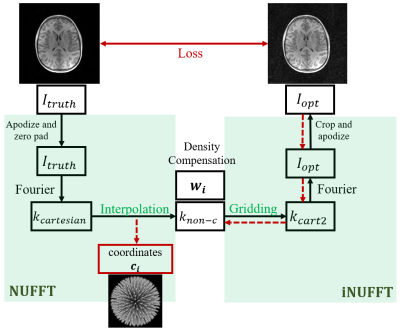 |
5 | Learned 3D radial trajectories using Image Quality Metrics from Prior Data
Chenwei Tang1, Laura Burns Eisenmenger2, Steven Kecskemeti3, and Kevin Johnson1,2
1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3University of Wisconsin-Madison, Madison, WI, United States
3D radial sampling provides high levels of acceleration with insensitivity to motion. Methods of designing the radial projection angles and orders have been focused on improving the sampling uniformity, which is indirectly related to image quality. We propose a flexible framework to optimize the sampling directly based on image reconstruction metrics. We demonstrated the optimized trajectories were able to produce higher quality images in both simulations and scans.
|
||
5013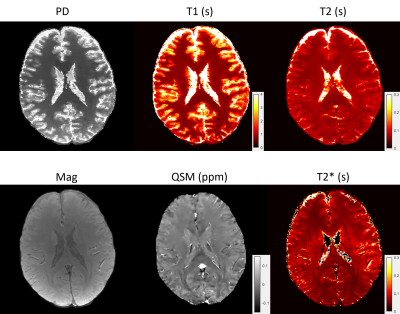 |
6 | High resolution 3D whole brain T1, T2, T2* and QSM mapping within 3.5 mins
Jinwei Zhang1, Thanh Nguyen2, Eddy Solomon2, Hang Zhang1, Chao Li1, Alexey Dimov2, Pascal Spincemaille2, and Yi Wang2
1Cornell University, New York, NY, United States, 2Weill Cornell Medicine, New York, NY, United States
A 3D whole brain T1, T2, T2*, QSM mapping pipeline was proposed for fast multi-parametric quantitative imaging. A multi-echo gradient echo (mGRE) sequence with varying sampling patterns along echoes was implemented to acquire signals for T2* and QSM mapping. A multi-contrast MR fingerprinting (MRF) sequence with both inversion recovery and T2 preparation pulses and varying sampling patterns among contrasts was implemented to acquire signals for T1 and T2 mapping. A deep ADMM network was used to reconstruct mGRE images. The magnitude image from reconstructed mGRE images was used to guide the reconstruction of MRF images with directional joint TV regularization.
|
||
5014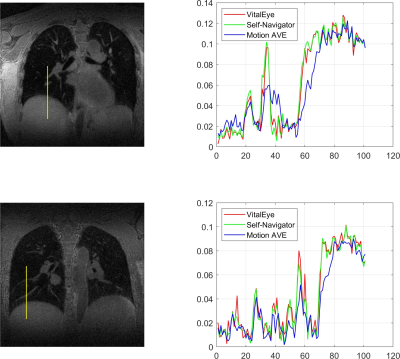 |
7 | Motion-Resolved 4D Radial Ultrashort Echo Time (UTE) Lung MRI with Built-in Camera-Based Respiratory Motion Sensing
Can Wu1, Guruprasad Krishnamoorthy2, Ergys Subashi1, Victoria Yu1, and Ricardo Otazo1,3
1Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Philips Healthcare, MR R&D, Rochester, MN, United States, 3Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Camera-based respiratory motion sensing (VitalEye) was successfully used to compute a respiratory signal for motion-resolved 4D radial ultrashort echo time (UTE) lung MRI. k-space data were sorted with respect the respiratory signal and binned into 10 different motion states to resolve respiratory motion. The respiratory signals from VitalEye were comparable to self-navigators. The 4D lung MRI reconstructions from both VitalEye and self-navigators were able to resolve respiratory motion and the signal intensity profiles along with the lung-liver interface and pulmonary vessels demonstrated that they both provided sharper image contrast compared to the motion-averaged images.
|
||
5015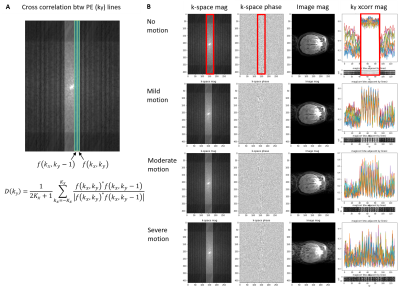 |
8 | Automated MRI k-space Motion Artifact Detection in Segmented Multi-Slice Sequences
Ikbeom Jang†1,2, Robert S Frost†1,2, Malte Hoffmann1,2, Nalini M Singh3,4, Lina Chen5, Arnaud Guidon6, Marcio Aloisio Bezerra Cavalcanti Rockenbach5, Donnella S Comeau5, Bernardo C Bizzo5, Ken Chang1,4, Sage Witham6, Dan Rettmann6, Suchandrima Banerjee6,
Anja Brau6, Timothy G Reese1,2, Iman Aganj1,2, Adrian Dalca1,2,3, Bruce Fischl*1,2,3,4, and Jayashree Kalpathy-Cramer*1,2,5
1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Boston, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Computer Science and Artificial Intelligence Laboratory, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Harvard-MIT Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 5MGH & BWH Center for Clinical Data Science, Mass General Brigham, Boston, MA, United States, 6GE Healthcare, Chicago, IL, United States
Motion artifacts negatively impact diagnosis and the radiology workflow, especially in cases where a patient recall is required. Detecting motion artifacts while the patient is still in the scanner could potentially improve workflow and reduce costs by enabling efficient corrective action. We introduce an algorithm that detects motion artifacts directly from raw k-space in a supervised learning manner in clinically important 2D FSE multi-slice scans, using cross-correlation between adjacent phase-encoding lines as features. This study employs a training approach that uses a motion simulator to generate k-space data with varying levels of motion artifact.
|
||
5016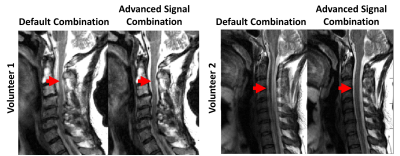 |
9 | Advanced Signal Combination for Improved Image Quality in the Presence of Motion
Hassan Haji-valizadeh1, Sampada Bhave1, Jennifer Wagner1, and Samir Sharma1
1Canon Medical Research USA, Inc., Mayfield Village, OH, United States
Motion is a major challenge in MRI. Averaging multiple acquisitions of the same target can help suppress motion artifacts. However, combining multiple acquisitions with equal weighting can produce non-diagnostic image quality if one acquisition suffers from significantly more motion artifacts than the other acquisition. For this study, a framework for suppressing motion artifacts was developed by assigning higher weighting to the acquisition with less motion. The performance of the proposed strategy, called advanced signal combination (ASC), was evaluated in sagittal T2-weighted C-spine MRI obtained with number of acquisitions=2. ASC was found to suppress motion artifact while maintaining SNR.
|
||
| 5017 | 10 | Artifact-specific MRI quality assessment with multi-task model
Ke Lei1, Avishkar Sharma2, Cedric Yue Sik Kin2, Xucheng Zhu3, Naeim Bahrami3, Marcus Alley2, John M. Pauly1, and Shreyas S. Vasanawala2
1Electrical Engineering, Stanford University, Stanford, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3GE Healthcare, Menlo Park, CA, United States
We present a CNN model that assesses diagnostic quality of MRIs in terms of noise, rigid motion and peristaltic motion artifacts. Our multi-task model consists of a set of shared convolutional layers followed by three branches, one for each artifact. We use dual-task training for two branches. We then utilize transfer learning for the third branch for which training data is scarce. We show that multi-task training improves the generalizability of the model, and transfer learning significantly improves the performance of the branch under data scarcity. Our model is deployed in clinically to provide warnings and artifact-specific solutions to technologists.
|
||
5018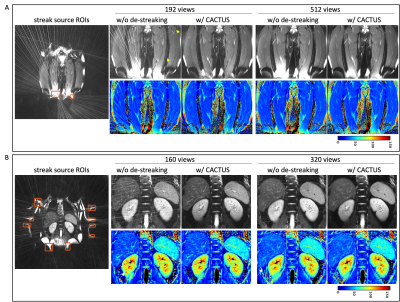 |
11 | Streak reduction in radial imaging with CACTUS
Zhiyang Fu1,2, Kevin Johnson1, Ali Bilgin1,2,3, and Maria I Altbach1,3
1Medical Imaging, The University of Arizona, Tucson, AZ, United States, 2Electrical and Computer Engineering, The University of Arizona, Tucson, AZ, United States, 3Biomedical Engineering, The University of Arizona, Tucson, AZ, United States
Radial trajectories are appealing for efficient parameter mapping due to their robustness to undersampling compared to Cartesian trajectories. However, streaks due to scanner imperfections can significantly affect image quality and accuracy of parameter maps. Previously, we developed a streak reduction technique named CACTUS. CACTUS was demonstrated in cross-sectional (axial) abdominal parameter imaging. The other two imaging planes (coronal and sagittal) in radial MRI are more vulnerable to these anomaly streaks. In this work, we demonstrate the benefits of CACTUS in all three imaging orientations and investigate the effect of undersampling on the presence of streaks and associated parameter maps.
|
||
5019 |
12 | Fast Distortion-Free Structural Imaging near Metal at 7T
Michael Mullen1, Michael Garwood1, and Essa Yacoub1
1Center for Magnetic Resonance Research and Department of Radiology, University of Minnesota, Minneapolis, MN, United States
Clinical MRI sequences for imaging near metallic implants are mainly multi-spectral approaches, with fast spin-echo acquisitions and large acceleration factors to achieve clinically relevant scan times. The authors previously reported a broadband, low flip angle method at 1.5T and 3T to image with large field inhomogeneity, such as near metallic implants, quickly relative to non-spatially selective multispectral approaches. Herein, modifications to the previous approach, dual polarity missing pulse steady-state free precession, are presented which achieve high spatial resolutions at 7T with a large 3D FOV in ~5 minutes.
|
||
5020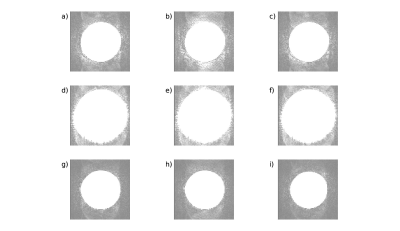 |
13 | Spikes-on-demand. A simple method to generate realistic spikes to test artifact detection algorithms
Jagjit Sidhu1, Ken Sakaie1, Ajay Nemani1, and Mark Lowe1
1Cleveland Clinic, Cleveland, OH, United States
Quality assurance (QA) protocols can and should be used to proactively detect low-level spiking and other artifacts early so that these problems can be remedied before becoming more debilitating and adding significant overhead to the image analysis workflow. However, one generally needs corrupted data sets to test the accuracy of the novel algorithm. This prevents researchers from taking a proactive stance of establishing these QA protocols before corruption of data sets occurs. Here, we detail a simple method to generate spikes reliably and reproducibly to generate corrupted data that can be used to test and debug any new QA algorithms.
|
||
5021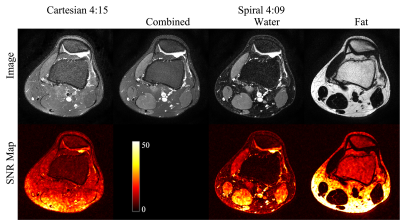 |
14 | Feasibility of Spiral Routine Knee Imaging at 1.5 T
Dinghui Wang1, Tzu-Cheng Chao1, Francis I. Baffour1, Guruprasad Krishnamoorthy2, and James G. Pipe1
1Department of Radiology, Mayo Clinic, Rochester, MN, United States, 2MR R&D, Philips Healthcare, Rochester, MN, United States
Spiral spin-echo and dual spin-echo sequences have been implemented for knee imaging at 1.5T with in-plane resolution of 0.51 by 0.51 mm2. High-quality proton density-weighted, T2-weighted and T1-weighted water and fat images at various orientations can be obtained using spiral Dixon imaging with comparable total scan time as the conventional Cartesian fast (turbo) spin-echo sequences without Dixon. Spiral T2-weighted water images demonstrated better fat suppression and a 24-67% increase of signal-to-noise ratio compared to fat-suppressed Cartesian reference images.
|
||
5022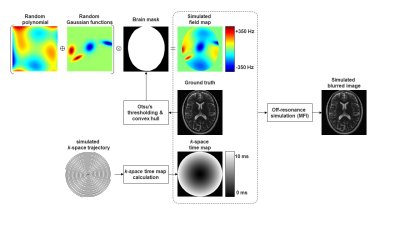 |
15 | Automatic Off-Resonance Correction for Spiral Imaging with a Convolutional Neural Network
Quan Dou1, Zhixing Wang1, Xue Feng1, and Craig H. Meyer1
1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States
Off-resonance is a major limitation for spiral imaging. A convolutional neural network was implemented in this study to correct off-resonance artifacts without field maps. The network was trained on images with simulated blurring artifacts. The image quality was improved after the correction for both simulated data and in vivo data.
|
||
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.