Oral Session
Data Analysis in the Brain
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

17:00 |
0160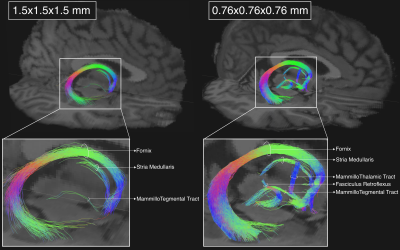 |
Submillimeter dMRI protocol optimization for accurate in-vivo reconstruction of deep-brain circuitry
Chiara Maffei1, Fuyixue Wang1, Suzanne Haber2,3, and Anastasia Yendiki1
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 2Department of Pharmacology and Physiology, University of Rochester School of Medicine, Rochester, NY, United States, 3McLean Hospital, Belmont, MA, United States The relatively low resolution of conventional in-vivo human dMRI prevents the reconstruction of small axonal bundles (diameter < 3mm). Here we show that diencephalic connections, which are inaccessible at macroscopic resolution (1.5mm), can be reconstructed with unprecedented accuracy at mesoscopic resolution (760μm). We investigate the minimum number of directions needed to achieve this with a state-of-the-art, multi-slab dMRI sequence. We find that a long, multi-session acquisition is necessary. We aim to develop a protocol for manual annotation of these bundles in a small number of high-quality datasets, and thus produce a training set for automated reconstruction in lower-quality dMRI data. |
|
17:12 |
0161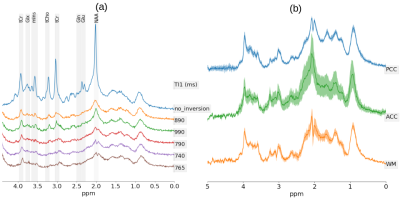 |
Macromolecule modelling for improved metabolite quantification using very short echo time MRS at 3T: The PRaMM model
Andrea Dell'Orco1,2,3,4, Layla Tabea Riemann2, Semiha Aydin2, Michael Scheel1, and Ariane Fillmer2
1Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt- Universität zu Berlin, Department of Neuroradiology, Berlin, Germany, 2Physikalisch-Technische Bundesanstalt (PTB), Braunschweig und Berlin, Germany, 3Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Department of Neurology, Berlin, Germany, 4Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, NeuroCure Clinical Research, Berlin, Germany
The accurate quantification of ultra-short echo-time 1H-MRS spectra is challenging due to broad macromolecular (MM) signal components. Here, we propose the parameterized-ratio MM (PRaMM) method to model the MM and its use in linear-combination model fitting of 1H-MRS spectra. The PRaMM method uses ratios of MM signal amplitudes as soft-constraints to limit the degrees of freedom of the fitting model, while allowing for more flexibility than commonly used approaches. The suggested model is demonstrated to improve the quantification of metabolites compared to two common methods for MM treatment.
|
|
17:24 |
0162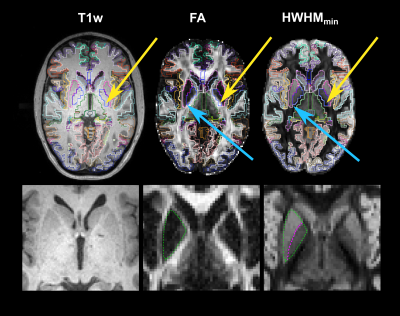 |
In vivo demonstration of generalized anisotropy profiles for resolving boundaries between subcortical gray and white matter
Robert Jones1, Chiara Maffei1, Qiyuan Tian1, Susie Huang1, Vaanathi Sundaresan1, and Anastasia Yendiki1
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital & Harvard Medical School, Charlestown, MA, United States
We investigate the use of generalized anisotropy profiles (GAPs) for delineating boundaries between subcortical gray and white matter in vivo. Replicating results from a previous ex vivo study, we show that GAPs, which are computed from the diffusion propagator, provide more informative contrasts than T1- and T2-weighted images or conventional diffusion metrics. An undersampled Cartesian-grid sampling of q-space can be used to obtain these profiles with a reasonable scan time. Thus, GAPs show promise as a multi-channel contrast that could be used in the future to improve structural segmentation.
|
|
| 17:48 | 0163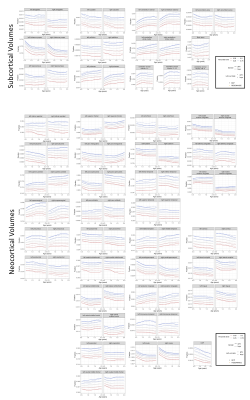 |
Longitudinal Brain and Cognitive Development of the First 1000 Days:A Large Multi-Cohort Multi-Scanner Study
Muriel Bruchhage1,2,3, Yaqing Chen4, Alvaro Gajardo Cataldo4, Hans-Georg Müller4, Elizabeth Weisee3, Sian Wilson5,6, Maximilian Pietsch5,7, Viren D'Sa2,3, Andre Marquand8, Sylvia Madhow9,10, Kristofer Bouchard9,10, James H. Cole11,12, Francesca Biondo11,13, Jed Elison14, Jonathan OMuirchheartaigh5,6,7, and Sean C. L. Deoni2,3,15
1Psychology, Stavanger University, Stavanger, Norway, 2Pediatrics, Warren Alpert Medical School at Brown University, Providence, RI, United States, 3Diagnostic Imaging, Rhode Island Hospital, Providence, RI, United States, 4Statistics, UC Davis, Davis, CA, United States, 5Centre for the Developing Brain, Department of Perinatal Imaging and Health, King's College London, London, United Kingdom, 6Department of Forensic & Neurodevelopmental Sciences, King's College London, London, United Kingdom, 7MRC Centre for Neurodevelopmental Disorders, King's College London, London, United Kingdom, 8Radboud University, Maastricht, Netherlands, 9Scientific Data Division & Biological Systems and Engineering Division, LBNL, Berkeley, CA, United States, 10Helen Wills Neuroscience Institute & Redwood Center for Theoretical Neuroscience, UC Berkeley, Berkeley, CA, United States, 11Centre for Medical Image Computing, Computer Science, UCL, London, United Kingdom, 12Dementia Research Centre, Queen Square Institute of Neurology, UCL, London, United Kingdom, 13Department of Neuroimaging, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London, United Kingdom, 14Institute of Child Development, University of Minnesota, Minneapolis, MN, United States, 15Brown's Advanced Baby Imaging Lab, Rhode Island Hospital, Providence, RI, United States
The first 1000 days are essential for a child’s development, consisting of critical time windows for brain and cognitive development but also vulnerability. In this large multi-cohort study, we investigated typical development of 478 children with ≤6 timepoints across two different scanners with different acquisition protocols. PACE brain-for-age growth percentiles and regression slope functions with 95% pointwise confidence intervals identified critical windows of neurocognitive development. Our results could allow for more appropriate neurodevelopmental burden estimates across multi-scanner cohorts, and help identify primary risk factors and age-specific intervention impact.
|
|
| 18:00 | 0164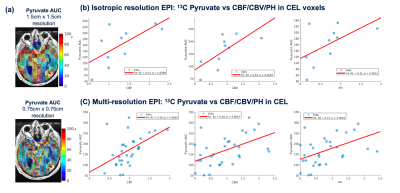 |
Evaluation of perfusion effects on HP[1-13C]pyruvate imaging parameters in patients with glioblastoma
Sana Vaziri1, Adam Autry1, Marisa Lafontaine1, Janine M Lupo1, Jeremy W Gordon1, Jasmine Hu1, Hsin-Yu Chen1, Yaewon Kim1, Javier Villanueva-Meyer1, Susan M Chang2, Jennifer Clarke2,3, Nancy Ann Oberheim Bush2,3, Duan Xu1, Peder EZ Larson1, Daniel B Vigneron1,4, and Yan Li1
1Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA, United States, 3Department of Neurology, University of California, San Francisco, San Francisco, CA, United States, 4Department of Bioengineering and Therapeutic Science, University of California, San Francisco, San Francisco, CA, United States
Dynamic hyperpolarized (HP) 13C metabolic imaging allows for non-invasive measurements of real-time enzymatic conversion of injected [1-13C]pyruvate to [1-13C]lactate. The HP signal depends on several factors, including perfusion and monocarboxylate transporter activity. Previously, a global increase of pyruvate-to-lactate apparent conversion rates values was found in patients with glioma after receiving anti-angiogenic therapies. To better understand the effects of tissue vasculature on HP signals, we investigated the relationship between HP[1-13C]pyruvate metabolism and 1H perfusion parameters in normal-appearing white matter patients with glioma.
|
|
18:12 |
0165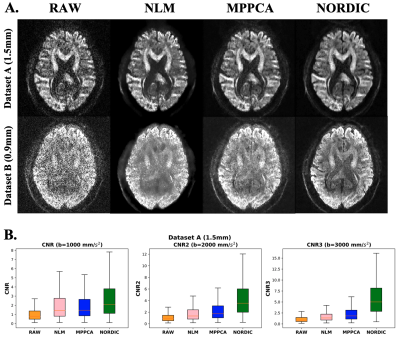 |
EDDEN: Towards a framework for Evaluating Diffusion MRI DENoising approaches
Jose Pedro Manzano Patron1,2, Steen Moeller3, Essa Yacoub3, and Stamatios Sotiropoulos1
1Sir Peter Mansfield Imaging Centre, Mental Health and Clinical Neurosciences, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 2Precision Imaging Beacon, University of Nottingham, Nottingham, United Kingdom, 3Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States Denoising diffusion MRI data has gained significant interest over the last years, due to the inherently low-SNR in the dMRI signal. Despite the existence of a number of denoising algorithms, open questions exist on how, and even whether, to denoise data. A consistent set of evaluations that comprehensively characterise newly-developed approaches and their impact on downstream applications is lacking for providing insight to these questions. Here we propose EDDEN, a framework for Evaluating DMRI DENoising approaches, consisting of a set of unique data and assessments. We demonstrate its use using 3 exemplar denoising methods (NLM, MPPCA and NORDIC). |
|
18:24 |
0166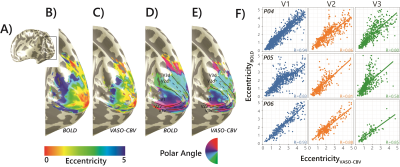 |
Comparing population Receptive Field mapping using VASO-CBV and BOLD
Icaro A.F. Oliveira1,2, Yuxuan Cai1,2, Shir Hofstetter1, Jeroen C.W. Siero1,3, Wietske van der Zwaag1, and Serge O. Dumoulin1,2,4
1Spinoza Centre for Neuroimaging, Amsterdam, Netherlands, 2Experimental and Applied Psychology, VU University, Amsterdam, Netherlands, 3Radiology, University Medical Centre Utrecht, Utrecht, Netherlands, 4Experimental Psychology, Helmholtz Institute, Utrecht University, Utrecht, Netherlands
We extended the use of VASO-CBV to pRF mapping modeling. We show that VASO-CBV data can be used reliably to map polar angle and eccentricity, similar to BOLD-based data. In addition, the pRF size increased systematically from V1 to V3 similarly for BOLD and VASO-CBV estimates. The higher microvascular specificity of VASO-CBV did not result in smaller pRF size estimates. This result suggests that the vascular contribution to the pRF size is not dominant in either VASO-CBV or BOLD-based pRF mapping.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.