Oral Session
Deep/Machine Learning-Based Image Acquisition & Reconstruction
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

09:15 |
0048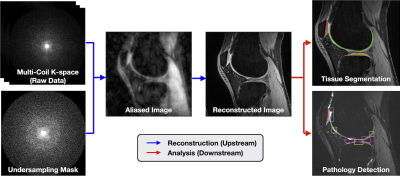 |
SKM-TEA: A Dataset for Accelerated MRI Reconstruction with Dense Image Labels for Quantitative Clinical Evaluation
Arjun D Desai1,2, Andrew M Schmidt2, Elka B Rubin2, Christopher M Sandino1, Marianne S Black2, Valentina Mazzoli2, Kathryn J Stevens2, Robert Boutin2, Christopher Ré3, Garry E Gold2,4, Brian A Hargreaves1,2,4, and Akshay S Chaudhari2,5
1Electrical Engineering, Stanford University, Stanford, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3Computer Science, Stanford University, Stanford, CA, United States, 4Bioengineering, Stanford University, Stanford, CA, United States, 5Biomedical Data Science, Stanford University, Stanford, CA, United States While deep-learning-based MRI reconstruction and image analysis methods have shown promise, few have been translated to clinical practice. This may be a result of (1) paucity of end-to-end datasets that enable comprehensive evaluation from reconstruction to analysis and (2) discordance between conventional validation metrics and clinically useful endpoints. Here, we present the Stanford Knee MRI with Multi-Task Evaluation (SKM-TEA), a dataset of 155 clinical quantitative 3D knee MRI scans with k-space data, DICOM images, and dense tissue segmentation and pathology annotations to facilitate clinically relevant, comprehensive benchmarking of the MRI workflow. Dataset, code, and trained baselines are available at https://github.com/StanfordMIMI/skm-tea. |
|
| 09:27 | 0049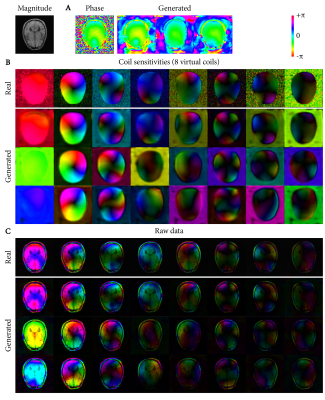 |
Deep-learning-based raw data generation for deep-learning-based image reconstruction
Frank Zijlstra1,2 and Peter T. While1,2
1Department of Radiology and Nuclear Medicine, St. Olav's University Hospital, Trondheim, Norway, 2Department of Circulation and Medical Imaging, NTNU - Norwegian University of Science and Technology, Trondheim, Norway
This study demonstrates the potential of using deep learning for generating raw data to augment raw datasets of limited size for use in deep-learning-based MR image reconstruction. Using an adversarial autoencoder architecture, variability in 10 T1-weighted raw datasets from the fastMRI database was learned and recombined into new, random raw data. We trained a deep-learning-based Compressed Sensing reconstruction using both conventional approaches with real raw data and compared the results to training with generated raw data. The reconstruction from generated raw data showed improved reconstruction results and better generalization to scans with slightly different echo times.
|
|
09:39 |
0050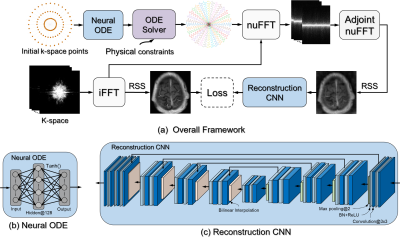 |
Learning Optimal K-space Acquisition and Reconstruction using Physics-Informed Neural Networks
Wei Peng1, Li Feng2, Guoying Zhao1, and Fang Liu3
1Computer Science and Engineering, University of Oulu, Oulu, Finland, 2Diagnostic, Molecular and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Radiology, Harvard Medical School, Boston, MA, United States
This work proposes a novel optimization framework to learn k-space sampling trajectories using deep learning by considering it an Ordinary Differential Equation (ODE) problem that can be solved using neural ODE. Particularly, the sampling of k-space data is framed as a dynamic system, in which neural ODE is formulated to approximate the system with additional constraints on MRI physics. Experiments were conducted on different in-vivo datasets (e.g., brain and knee images) acquired with different sequences. Initial results have shown that our proposed method can generate better image quality in accelerated MRI than conventional undersampling schemes in Cartesian and non-Cartesian acquisitions.
|
|
09:51 |
0051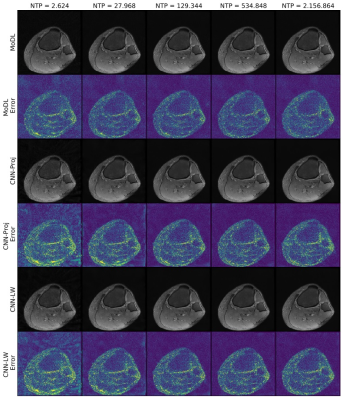 |
The More the Merrier? - On the Number of Trainable Parameters in Iterative Neural Networks for Image Reconstruction
Andreas Kofler1, Tobias Schaeffter1,2,3, and Christoph Kolbitsch1,3
1Physikalisch-Technische Bundesanstalt, Braunschweig and Berlin, Germany, 2Department of Biomedical Engineering, Technische Universität Berlin, Berlin, Germany, 3School of Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom
Iterative neural networks (INNs) currently define the state-of-the-art for image reconstruction methods. With these methods, the obtained regularizers are not only optimally adapted to the employed physical model but also tailored to the reconstruction method the network implicitly defines. However, comparing the performance of different INNs-based methods is often challenging because of the black-box character of neural networks. In this work we construct an example which highlights the importance of keeping the number of trainable parameters approximately fixed when comparing INNs-based methods. If this aspect is not taken into consideration, wrong conclusions could be drawn.
|
|
| 10:03 | 0052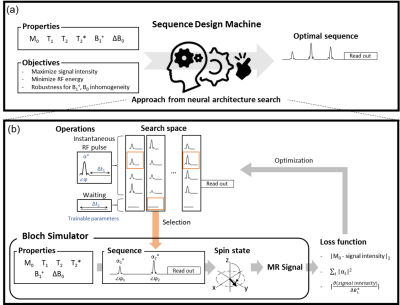 |
Automated Sequence Design using Neural Architecture Search
Hongjun An1, Sooyeon Ji1, Sehong Oh2, Jiye Kim1, Dongmyung Shin1, and Jongho Lee1
1Department of Electrical and Computer Engineering, Seoul National University, Seoul, Korea, Republic of, 2Division of Biomedical Engineering, Hankuk University of Foreign Studies, Gyeonggi-do, Korea, Republic of
A new pulse sequence design method that requires no prior knowledge of MR physics or training dataset is proposed. This method utilizes neural architecture search, generating an optimal sequence for given properties (e.g., T2*, B1+) and target objectives. It successfully discovered FLAIR-like and spin-echo-like sequences. Furthermore, a less-intuitive sequence using three RF pulses was created when targeted for spin-echo. In an in-vivo experiment, this new sequence had almost identical contrasts and SNR with spin-echo (our SNR: 297.1±70.1; spin-echo SNR: 295.8±78.2) while utilizing 90% of RF energy of the spin-echo, suggesting a potential of the automated sequence design.
|
|
| 10:15 | 0053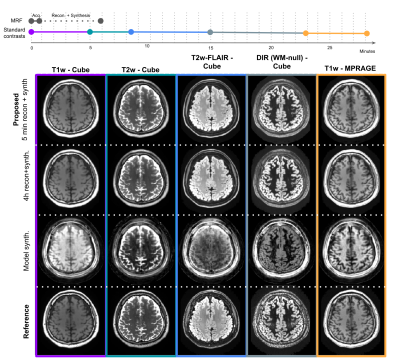 |
Toward a 1-minute high-resolution brain exam - MR Fingerprinting with fast reconstruction and ML-synthesized contrasts
Sophie Schauman1, Siddharth Srinivasan Iyer1,2, Mahmut Yurt1, Xiaozhi Cao1, Congyu Liao1, Zheng Zhong1, Guanhua Wang3, Greg Zaharchuk1, Shreyas Vasanawala1, and Kawin Setsompop1
1Department of Radiology, Stanford University, Stanford, CA, United States, 2Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Deptartment of Biomedical Engineering, University of Michigan, Ann Arbor, MI, United States
MRI is a notoriously slow imaging method, but in recent years many technical developments have improved acquisition speed. However, many of these new fast sequences have not made their way to clinical practice. Barriers to uptake include long reconstruction times, diminished image quality, and non-standard contrast in the resulting images. Our objective is to translate a ~1 minute MRF sequence into clinical practice, providing five high quality images with common clinical contrasts at 1 mm isotropic-resolution, as well as quantitative T1, T2, and proton density maps, all within a 5 minute reconstruction pipeline that we have deployed clinically.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.