Oral Session
Quantitative Relaxation Parameter Mapping in the Body
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| 14:45 | 0109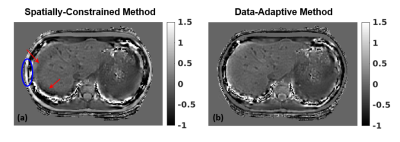 |
Accuracy, Repeatability, and Reproducibility of Regularized Inversions for Abdominal Quantitative Susceptibility Mapping Video Not Available
Julia Velikina1, Ruiyang Zhao1,2, Collin J Buelo1,2, Alexey A Samsonov1, Scott B Reeder1,2,3,4, and Diego Hernando1,2
1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 3Medicine, University of Wisconsin-Madison, Madison, WI, United States, 4Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Quantitative susceptibility mapping (QSM) is a promising non-invasive technique for assessment of liver iron concentration (LIC), necessary in a number of diseases. QSM solves an ill-conditioned inverse problem, whose performance depends on the chosen regularization. This work is the first to evaluate the accuracy, repeatability, and reproducibility of liver QSM for two regularized inversion methods in a large patient population with a wide range of LIC. Our results indicate that data-adaptive regularization shows higher correlation with reference LIC values and increases repeatability/reproducibility due to its reduced sensitivity to the field map errors and regularizing effect of anatomical priors.
|
|
| 14:57 | 0110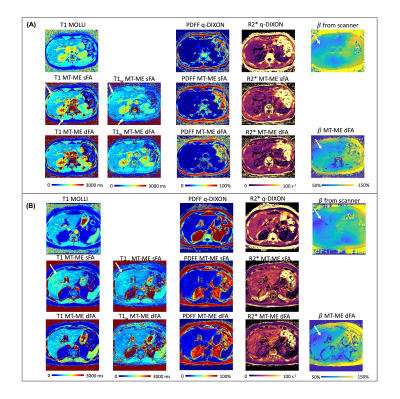 |
Free-Breathing Dual-Flip-Angle Multitasking Multi-Echo (MT-ME-dFA) MRI for Whole-Liver, B1+ Insensitive Quantification of T1, PDFF, and R2*
Nan Wang1, Fardad M. Serry2, Tianle Cao2, Fei Han3, Yibin Xie2, Xiaodong Zhong3, Sen Ma2, Xiaoming Bi3, Mazen Noureddin2, Shehnaz Hussain4, Vibhas Deshpande5, Anthony G. Christodoulou2, and Debiao Li2
1Stanford University, Stanford, CA, United States, 2Cedars-Sinai Medical Center, Los Angeles, CA, United States, 3Siemens Medical Solutions, Los Angeles, CA, United States, 4University of California, Davis, Davis, CA, United States, 5Siemens Medical Solutions, Austin, TX, United States
Quantitative T1, fat fraction, and R2* show promise for characterizing chronic liver diseases. We develop a dual-flip-angle MultiTasking Multi-Echo (MT-ME-dFA) technique to achieve B1+ insensitive, whole-liver joint T1/water-specific T1(T1w), fat fraction(PDFF), and R2* quantification. Respiratory motion is resolved through multidimensional low-rank tensor imaging, and B1+ robustness is achieved by modeling the inversion recovery with two alternating flip angles. Compared to the previously published single-flip-angle MT-ME approach, intra-subject variation on T1/T1w maps was substantially reduced; stronger correlation was achieved for T1/T1w, PDFF and R2* with references on phantom; root-mean-square of intra-subject T1/T1w variation was substantially reduced from 72ms/63ms to 39ms/31ms.
|
|
| 15:09 | 0111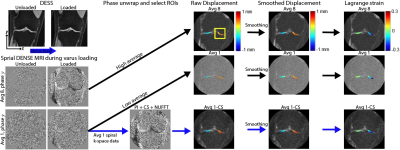 |
In Vivo High Frame Rate Cartilage Strain During Knee Varus Combining Spiral DENSE MRI and Compressed Sensing
Woowon Lee1, Emily Y Miller2, Hongtian Zhu1, and Corey P Neu1,2
1Paul M. Rady Department of Mechanical Engineering, University of Colorado, Boulder, CO, United States, 2Biomedical Engineering Program, University of Colorado, Boulder, CO, United States
Displacement encoding with stimulated echoes (DENSE) MRI is used to calculate pixel-level deformation maps of soft tissues under repetitive motion. It is a powerful technique that can quantify the mechanical behavior of tissues from displacement. We apply spiral DENSE MRI on human knees and obtain multi-frame displacement and strain maps during varus loading, leading to compressive motion on the medial condyle. Since high SNR DENSE images require long scanning time which is costly and less tolerable for participants, we additionally apply compressed sensing (CS) to reduce the imaging time to less than five minutes.
|
|
| 15:21 | 0112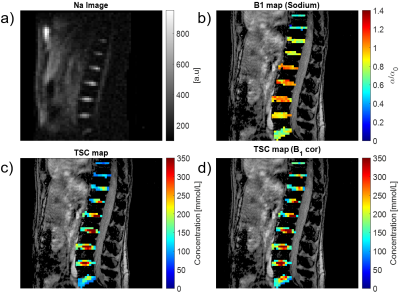 |
Quantitative 23Na-MRI of the intervertebral disk at 3T Video Not Available
Mustafa Cavusoglu1 and Christina Rossi2
1University of Zurich, Zurich, Switzerland, 2University Hospital Zurich, Zurich, Switzerland Monitoring the tissue sodium content (TSC) in the intervertebral disk geometry by MRI is sensitive measure to diagnose degenerative disk disease (DDD) and of lumbar back pain (LBP) in intervertebral disks. However, application of quantitative sodium concentration measurements in 23Na-MRI is highly challenging due to the lower in vivo concentrations and smaller gyromagnetic ratio. Moreover, imaging the intervertebral disk geometry places higher demands because the necessary RF volume coils produces highly inhomogenous transmit field patterns. In this study, we reported for the first time quantitative sodium concentration in the intervertebral disks at clinical field strengths (3T). |
|
| 15:33 | 0113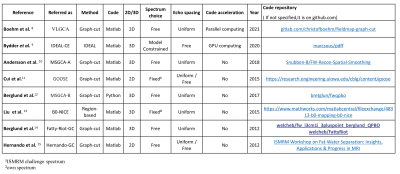 |
Comparative Review of Algorithms and Methods for proton-density fat fraction (PDFF) quantification
Pierre Daudé1,2, Frank Kober1,2, Sylviane Confort Gouny1,2, Monique Bernard1,2, and Stanislas Rapacchi1,2
1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France
An open-source toolbox has been implemented to compare state-of-the-art open-source fat-water separation algorithms over synthetic multi-echo data. Data varied in fat-fraction, B0, SNR, number of echoes and echo spacings. Most algorithms proved to be biased for 3 echoes data. For 5 echoes and more, six algorithms were comparable, but two algorithms proved to be inaccurate. Echo spacing scheme impacted quantitative limits of agreements. For proton-density fat-fraction(PDFF) quantification in the extreme ranges, graph-cut approaches provided similar results while IDEAL-CE provided more reliable results. Interestingly, the toolbox also revealed PDFF/T2* quantification to be sensitive to the choice of the fat spectrum.
|
|
| 15:45 | 0114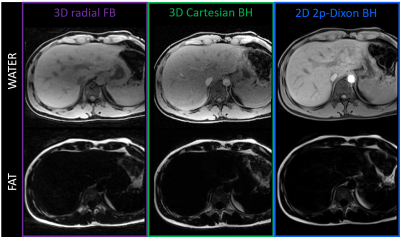 |
Pilot Tone-guided focused navigation for free-breathing whole-liver fat-water quantification
Adèle LC Mackowiak1, Christopher W Roy1, Mariana BL Falcaõ1, Aurélien Bustin1,2,3, Mario Bacher1,4, Peter Speier4, Davide Piccini1,5, Matthias Stuber1,6, Naïk Vietti-Violi1, and Jessica AM Bastiaansen1,7,8
1Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 3IHU LIRYC, Electrophysiology and Heart Modeling Institute, INSERM U1045, Centre de Recherche Cardio-Thoracique de Bordeaux, Université de Bordeaux, Bordeaux, France, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 6CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 7Department of Diagnostic, Interventional and Pediatric Radiology (DIPR), Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland, 8Translational Imaging Center, sitem-insel, Bern, Switzerland
A free-breathing 3D radial multi-echo GRE acquisition for whole-liver fat-water separation and quantification was proposed, which integrated retrospective respiratory motion extraction with Pilot Tone and motion-compensated reconstruction with focused navigation. The proposed framework was tested in 10 healthy volunteers at 1.5T. Post-processing of the 8 reconstructed and denoised echoes with a graphcut algorithm provided fat-water separated images and fat fraction maps of isotropic resolution. Images and maps were compared to breath-held reference 3D and 2D Cartesian acquisitions for validation of the quality of both motion compensation and fat-water separation.
|
|
15:57 |
0115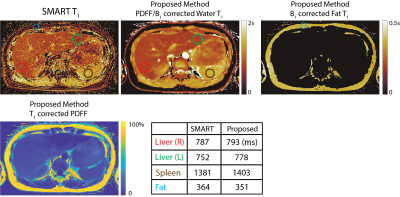 |
Free-Breathing, Fat- and B1-corrected T1 Mapping of the Liver with Chemical Shift Encoded Inversion Recovery MRI
Yavuz Muslu1,2, Ty A. Cashen3, Sagar Mandava4, and Scott B. Reeder1,2,5,6,7
1Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3GE Healthcare, Waukesha, WI, United States, 4GE Healthcare, Atlanta, GA, United States, 5Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Medicine, University of Wisconsin-Madison, Madison, WI, United States, 7Department of Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
T1 relaxation is emerging as a quantitative biomarker for the diagnosis and staging of chronic liver disease. Conventional T1 mapping methods such as variable flip angle and Modified Look-Locker Inversion Recovery, fail to address confounding factors such as B1 inhomogeneities, the presence of fat, and motion. In this work, we propose a novel free-breathing T1 mapping method that addresses multiple confounding factors, combining chemical shift encoded MRI, inversion recovery and stack-of-stars sampling strategy for abdominal T1 mapping.
|
|
| 16:09 | 0116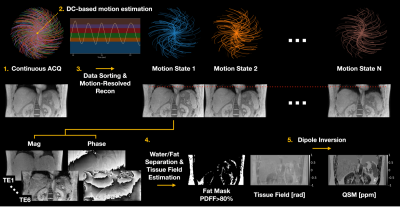 |
Free-Breathing Liver QSM with High Isotropic Resolution Using Respiratory Motion-Resolved 3D Multi-Echo Cones MRI
MungSoo Kang1,2, Ramin Jafari1, Gerald G. Behr3, Ricardo Otazo1,3, and Youngwook Kee1
1Department of Medical Physics, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Department of Biomedical Engineering, Ulsan National Institute of Science and Technology, Ulsan, Korea, Republic of, 3Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States
Liver quantitative susceptibility mapping (QSM) can provide a direct measurement of liver iron concentration (LIC). However, respiratory motion and rapid T2* decay are the major challenges in reliable liver QSM. To address these issues a motion-resolved multi-echo 3D cones MRI method with pseudo-random view ordering was implemented, which was then compared with a multi-echo Cartesian GRE sequence in a phantom and healthy volunteer. The proposed motion-resolved cones QSM presented strong motion-robustness in terms of image quality and ROI-based measurements in our phantom and in-vivo studies, demonstrating the feasibility of free-breathing liver QSM.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.