Oral Session
MRS & MRSI
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| 09:15 | 0054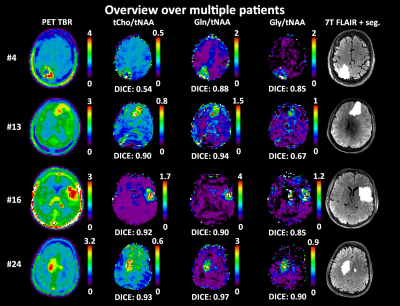 |
Comparing high resolution 7T MRSI and PET of glioma – a promising correspondence of glutamine to amino acid PET
Gilbert Hangel1, Philipp Lazen2, Sukrit Sharma2, Barbara Hritoska3, Cornelius Cadrien1, Julia Furtner4, Ivo Rausch5, Tatjana Traub-Weidinger6, Eva Niess2, Lukas Hingerl2, Stephan Gruber2, Bernhard Strasser2, Barbara Kiesel7, Matthias Preusser8, Thomas Roetzer-Pejrimovsky9, Adelheid Woehrer9, Wolfgang Bogner2, Georg Widhalm7, Karl Rössler7, and Siegfried Trattnig2,10,11
1Department of Neurosurgery & High Field MR Centre, Medical University of Vienna, Wien, Austria, 2High Field MR Centre, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 3FH Campus Wien University of Applied Sciences, Vienna, Austria, 4Division of Neuroradiology and Musculoskeletal Radiology, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 5Center for Medical Physics and Biomedical Engineering,, Medical University of Vienna, Vienna, Austria, 6Division of Nuclear Medicine, Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 7Department of Neurosurgery, Medical University of Vienna, Vienna, Austria, 8Division of Oncology, Department of Internal Medicine I, Medical University of Vienna, Vienna, Austria, 9Division of Neuropathology and Neurochemistry, Department of Neurology, Medical University of Vienna, Vienna, Austria, 10Institute for Clinical Molecular MRI, Karl Landsteiner Society, St. Pölten, Austria, 11Christian Doppler Laboratory for Clinical Molecular MR Imaging, Vienna, Austria We compared metabolic ratio maps of tCho, Gln, and Gly to tNAA and tCr derived from high-resolution 7T MRSI to clinical amino acid PET in 24 glioma patients. We achieved the highest DICE coefficient of 0.66±0.25 for Gln/tNAA, and further defined hot spot volumes and center of intensity distances between PET and MRSI. Overall, we showed that the information content of 7T MRSI results in a better correspondence to PET as clinical gold standard than previous MRSI studies. |
|
| 09:27 | 0055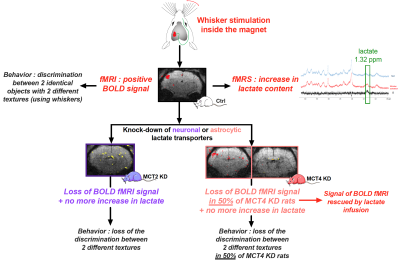 |
Importance of the lactate shuttle between astrocytes and neurons for brain activation
Hélène Roumes1, Charlotte Jollé2, Jordy Blanc1, Imad Benkhaled1,3, Philippe Massot1, Marc Biran1, Carolina Piletti Chatain4, Gérard Raffard1, Véronique Bouchaud1, Nicole Deglon5, Eduardo R Zimmer6, Luc Pellerin7, and Anne-Karine Bouzier-Sore1
1CRMSB, Bordeaux, France, 2Department of Physiology, Lausanne, Switzerland, 3I3M, Poitiers, France, 4Department of Biochemistry, Porto Alegre, Brazil, 5Laboratory of Cellular and Molecular Neurotherapies, Lausanne, Switzerland, 6Department of Pharmacology,, Porto Alegre, Brazil, 7IRTOMIT, Poitiers, France
For decades, it was claimed that glucose was the sole and sufficient energy substrate to sustain neuronal activity and brain function. Our results challenge this view by demonstrating that despite glucose availability, lactate shuttling from astrocytes to neurons via monocarboxylate transporters is necessary to give rise to the BOLD signal in the rat cerebral cortex following whisker stimulation. Moreover, lactate shuttling turned out to be also essential for sustaining behavioral performance associated with activation of the rat barrel cortex. These findings call for a reappraisal of neuroenergetics and the role of astrocytes in determining brain activation and function.
|
|
| 09:39 | 0056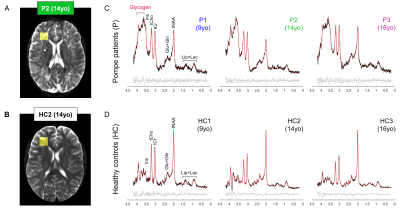 |
Glycogen accumulation in the brain of classic-infantile Pompe patient measured with single-voxel 1H MRS and 2D-MRSI at 7T
Chloé Najac1, Vincent O. Boer2, Nadine A.M.E. van der Beek3, Ans T. van der Ploeg4, Itamar Ronen1, Johanna M.P. van den Hout4, and Hermien E. Kan1
1C.J. Gorter Center for High Field MRI, Department of Radiology, Leiden, Netherlands, 2Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Amager and Hvidovre, Copenhagen, Denmark, 3Center for Lysosomal and Metabolic diseases, Department of Neurology, Erasmus MC University Medical Center, Rotterdam, Netherlands, 4Center for Lysosomal and Metabolic diseases, Department of Pediatrics, Erasmus MC University Medical Center, Rotterdam, Netherlands
Pompe disease is caused by an abnormal accumulation of glycogen in the lysosomes of multiple tissues including the brain due to a deficit in acid α-glucosidase (GAA). The development of enzyme replacement therapy with recombinant human GAA (rhGAA) has dramatically improved patients’ survival, however, rhGAA does not reach the brain which remains untreated. Consequently, classic-infantile Pompe patients may develop progressive white matter lesions and cognitive problems. Here, we used single-voxel 1H MRS and spectroscopic imaging and found an accumulation of glycogen and significant decrease in total-N-acetyl-aspartate in the brain of classic-infantile patients (n=3) when compared to age-matched healthy controls (n=3).
|
|
09:51 |
0057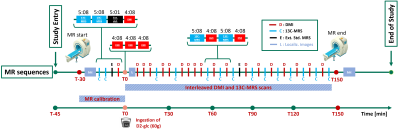 |
Interleaved 1H-MRI, 2H-MRSI and 13C-MRS for time-resolved in vivo elucidation of glucose metabolism in human liver at 7 T
Simone Poli1,2, Ahmed Fahiem Emara3, Edona Ballabani3, Angeline Buser3, Luc Tappy3, Lia Bally3, and Roland Kreis1,2
1Magnetic Resonance Methodology, Institute of Diagnostic and Interventional Neuroradiology, University of Bern, Bern, Switzerland, 2Translational Imaging Center, sitem-insel, Bern, Switzerland, 3Department of Diabetes, Endocrinology, Nutritional Medicine and Metabolism UDEM, Insel Hospital, University Hospital Bern, Bern, Switzerland
An interleaved 2H-MRSI / 13C-MRS protocol to obtain a complete picture of glucose turnover in the human liver at 7T is presented. A dedicated triple-tuned surface coil was used and the setup was optimized for temporal and spatial resolution, SNR (under the restraints of SAR) and robustness to assess hepatic glucose uptake and glycogen production. Data quality and initial kinetic data after oral deuterated glucose load is reported. Further optimization is intended to establish absolute concentrations and to generate kinetic models of metabolic fluxes.
|
|
| 10:03 | 0058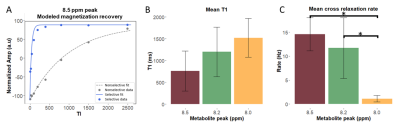 |
Quantification of cross-relaxation in human skeletal muscle using downfield 1H MRS at 7T
Sophia Swago1, Abigail Cember2, Puneet Bagga3, Neil Wilson2, Mark A. Elliott2, Ravi Prakash Reddy Nanga2, Ravinder Reddy2, and Walter Witschey2
1Department of Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 2Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 3St. Jude Children's Research Hospital, Memphis, TN, United States
In the 1H magnetic resonance (MR) spectrum of human muscle, non-exchangeable proton resonances have been observed at 8.0, 8.2 and 8.5 ppm. While relaxation rate enhancement of these resonances was previously observed, their cross-relaxation rates with bulk water have not yet been determined. Knowledge of cross-relaxation rates could improve the design of MR techniques or serve as a potential biomarker. We quantify the cross-relaxation rate of these resonances with bulk water using selective and non-selective inversion-recovery downfield MRS at 7T. We observed the cross-relaxation rates of the 8.2 and 8.5 ppm resonances were significantly faster than the 8.0 ppm resonance.
|
|
| 10:15 | 0059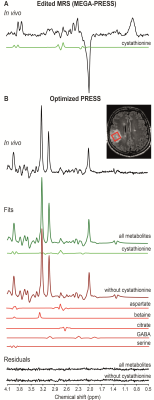 |
The influence of cystathionine on neurochemical quantification in brain tumor in vivo magnetic resonance spectroscopy
Francesca Branzoli1,2, Dinesh K Deelchand3, Roberto Liserre4, Pietro Luigi Poliani5, Lucia Nichelli6, Marc Sanson2,7, Stéphane Lehéricy1,2,6, and Małgorzata Marjańska3
1Center for Neuroimaging Research - CENIR, Paris Brain Institute - ICM, Paris, France, 2UMR S 1127, Inserm U 1127, CNRS UMR 7225, Sorbonne University, Paris, France, 3Center for Magnetic Resonance Research, Department of Radiology, University of Minnesota, Minneapolis, MN, United States, 4Department of Radiology, Neuroradiology Unit, ASST Spedali Civili University Hospital, Brescia, Italy, 5Pathology Unit, Department of Molecular and Translational Medicine, Brescia University, Brescia, Italy, 6Department of Neuroradiology, Pitié Salpêtrière hospital, Paris, France, 7Department of Neurology 2, Pitié Salpêtrière hospital, Paris, France
We reported the ability of PRESS to detect cystathionine in vivo in patients with glioma and the effect of the omission of cystathionine on the quantification of the full neurochemical profile. We showed that the omission of cystathionine from analysis leads to severe biases on the quantification of other metabolites involved in cancer metabolism, e.g., aspartate, betaine, citrate, GABA and serine. Because cystathionine was shown to accumulate preferentially in gliomas with 1p/19q codeletion, omission of this metabolite may lead to the wrong evaluation of other metabolic changes in these tumors.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.