Oral Session
CEST & MT
Joint Annual Meeting ISMRM-ESMRMB & ISMRT 31st Annual Meeting • 07-12 May 2022 • London, UK

| 16:45 | 0375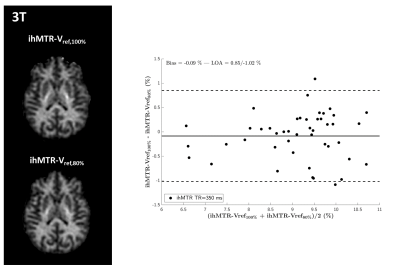 |
Inhomogeneous Magnetization Transfer (ihMT) imaging of the human brain at 7T
Olivier M. Girard1,2, Lucas Soustelle1,2, Thomas Troalen3, Andreea Hertanu1,2, Arash Forodighasemabadi1,2,4,5, Maxime Guye1,2, Jean-Philippe Ranjeva1,2, Gopal Varma6, David C. Alsop6, and Guillaume Duhamel1,2
1Aix Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3Siemens Healthcare SAS, Saint-Denis, France, 4Aix-Marseille Univ, Université Gustave Eiffel, LBA, Marseille, France, 5iLab-Spine International Associated Laboratory, Marseille - Montreal, France, 6Division of MR Research, Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
Inhomogeneous Magnetization Transfer (ihMT) MRI has raised interest for myelin imaging. Applications at ultra-high field are appealing for SNR considerations but are challenged by the reduction of available RF power due to regulatory SAR constraints and the need to account for inhomogeneities of the RF excitation field (B1+). In the current study we present experimental data acquired at 3T which validate two B1+-bias removal strategies. Then we further evaluate them at 7T and show preliminary results towards a robust ihMT protocol free from B1+ bias.
|
|
| 16:57 | 0376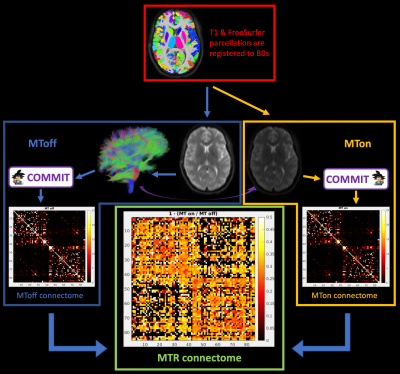 |
Feasibility of magnetization transfer measurement in distinct white matter connections
Pietro Bontempi1,2, Ilana R. Leppert3, Simona Schiavi1,4, Jennifer S. W. Campbell3, Mark Nelson3,5, Bruce G. Pike6, Christine L. Tardif3,5,7, and Alessandro Daducci1
1Department of Computer Science, University of Verona, Verona, Italy, 2Department of Molecular Biotechnology and Health Sciences, University of Turin, Turin, Italy, 3McConnell Brain Imaging Centre, Montreal Neurological Institute, McGill University, Montreal, QC, Canada, 4Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health (DINOGMI), University of Genoa, Genova, Italy, 5Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada, 6Hotchkiss Brain Institute and Departments of Radiology and Clinical Neuroscience, University of Calgary, Calgary, AB, Canada, 7Department of Biomedical Engineering, McGill University, Montreal, QC, Canada
Magnetization Transfer (MT) imaging is a MR technique that sensitizes image contrast to protons bound to macromolecules such as protein and lipids. Using an innovative sequence that combines MT and diffusion- imaging, and Convex Optimization Modeling for Microstructure Informed Tractography (COMMIT), a method that allows estimation of bundle tissue properties, we investigate bundle specific MT ratio (MTR) values.
|
|
| 17:09 | 0377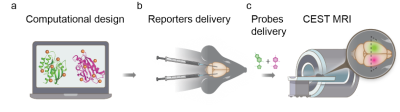 |
Genetically Engineered “Multicolour” MRI Reporters for In Vivo Imaging of Transgenes Expression
Hyla Allouche-Arnon1, Olga Khersonsky2, Nishanth Deva Tirukoti1, Liat Avram3, Talia Harris3, Nirbhay N. Yadav4,5, Sarel J. Fleishman2, and Amnon Bar-Shir1
1Department of Molecular Chemistry and Materials Science, Weizmann institute of science, Rehovot, Israel, 2Department of Biomolecular Sciences, Weizmann institute of science, Rehovot, Israel, 3Department of Chemical Research Support, Weizmann institute of science, Rehovot, Israel, 4Russell H. Morgan Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 5F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States Genetically engineered multicolor fluorescent proteins have changed biomedical research by enabling multiplexed mapping of transgenes expression. However, although MRI reporter genes have been developed, the ability to monitor multiple reporters that are expressed simultaneously and present them in a multicolor fashion is still needed. Here, we present an MRI-based system, comprising computationally designed reporters (enzymes) combined with MRI-detectable synthetic probes (substrates) for non-invasive color-encoded MRI mapping of transgene expression. Systemically administered reporter probes exclusively accumulate in cells expressing the designed reporter genes allowing their spatial display as pseudo-colored CEST MRI maps as demonstrated in both tumor model and viral-delivery system. |
|
17:21 |
0378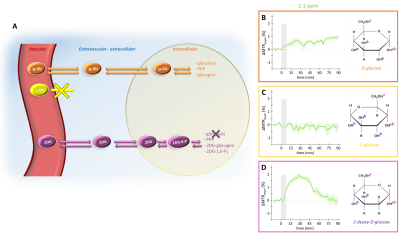 |
Deciphering the compartmental origin of glucoCEST signal using diffusion-weighted CEST-MRS
Yohann Mathieu-Daudé1, Mélissa Vincent1, Julien Valette1, and Julien Flament1
1Université Paris-Saclay, Commissariat à l’Energie Atomique et aux Energies Alternatives (CEA), Centre National de la Recherche Scientifique (CNRS), Molecular Imaging Research Center (MIRCen), Laboratoire des Maladies Neurodégénératives, Fontenay-aux-Roses, France
Compartmental origin of glucoCEST signal is still an ongoing debate. To address this crucial question, we propose in this study to use diffusion-weighted CEST-MRS in order to disentangle extracellular/extravascular and intracellular contributions to the glucoCEST signal. D-glucose and 2-deoxy-D-glucose were injected intravenously and kinetics of glucoCEST signal were measured over time. The comparison of kinetics acquired with and without diffusion grandients showed that 2-deoxy-D-glucose glucoCEST signal was dominated by intracellular contribution, contrarily of D-glucose. This study could constitute a major step toward glucoCEST quantification and could be beneficial for several applications, especially to study energy metabolism defects in neurodegenerative disorders.
|
|
| 17:33 | 0379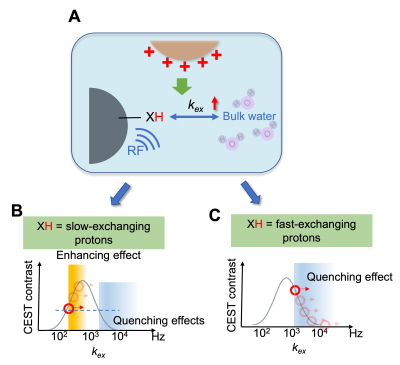 |
Magnetic resonance imaging of cationic compounds using their proton exchange modulating effect
Zheng Han1,2, Safiya Aafreen3, Yang Zhou1,2,4, Chongxue Bie1,2, Jiadi Xu1,2, Lei Zheng5, Peter C.M. van Zijl1,2, and Guanshu Liu1,2
1Radiology, Johns Hopkins University, Baltimore, MD, United States, 2F.M Kirby Research Center, Kennedy Krieger Institute, Baltimore, MD, United States, 3Biomedical Engineering, Johns Hopkins University, Baltimore, MD, United States, 4Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 5Oncology, Johns Hopkins University, Baltimore, MD, United States
Here we report a novel approach to detect non-labeled cationic compounds, named modulated saturation transfer (MOST)-MRI, based on their effect of modulating the exchange rate of surrounding exchangeable protons. MOST-MRI was able to detect polyethylenimine (PEI600, M.W. = 600 Da), at a high sensitivity (μM or μg/mL range) through its quenching effects on surrounding fast-exchanging protons. In animals, MOST-MRI detected the tumor uptake of PEI600 that was injected intratumorally at a low dose (50 μg/kg b.w.), indicating the potential of MOST-MRI for label-free imaging guidance that is needed for the development and clinical translation of cationic gene delivery systems.
|
|
| 17:45 | 0380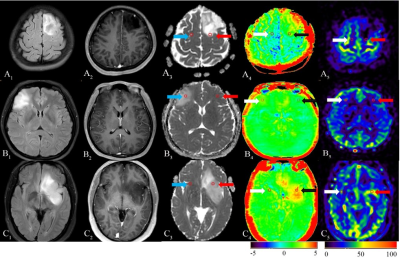 |
Diagnostic Performance of Diffusion Weighted Imaging, Arterial Spin Labeling and Amide Proton Transfer in sub-classifications of gliomas Video Not Available
DiaoHan Xiong1, Rui Wang1, Tao Wen1, TieJun Gan1, PengFei Wang1, XinYing Ren1, YuJing Li1, Jing Zhang1, and Kai Ai2
1Lanzhou University Second Hospital, Lanzhou, China, 2Philips Healthcare, Xi'an, China
To assess the diagnostic performance of Amide Proton Transfer (APT) and Arterial Spin Labeling (ASL) for distinguishing different sub-classifications of gliomas in comparison with Diffusion Weighted Imaging (DWI). Forty patients underwent sequences including T1W enhancement, DWI, APT and 3D-pCASL. ADCmean, ADCmean-to-ADCNAWM ratio, APTmean, APTmean-to-APTNAWM ratio, regional cerebral blood flow (rCBF) and rCBFmean-to-rCBFNAWM ratio are calculated. The results showed that diagnostic accuracy of APTmean was superior in distinguishing sub-classifications of gliomas than other parameters. We concluded that APT can be used in predicting sub-classification of gliomas based on WHO CNS5, providing more valuable supplementary information for early treatment guidance and surgery.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.